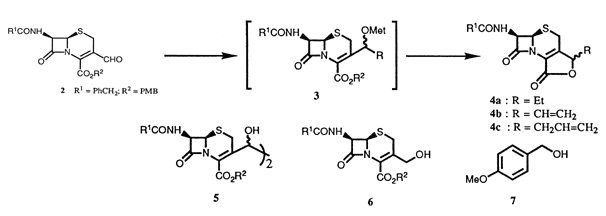
C(3)-Carbon Chain Elongation of Cephalosporins MXn/M' Bimetal Redox-Promoted Allylation of 3-Formylcephems
Tanaka, H.; Yamaguchi, T.; Taniguchi, M.; Kameyama, Y.; Sasaoka, M.; Shiroi, T.; Torii, S. Chem. Express 1991, 6, 435.
Elongation of C(3)-carbon chain of caphalosporins was performed by "Barbier Type" reaction of 3-formylcephem 2 with allyl halides and carbon tetrachloride in a metal salts (MXn)/metal (M')-bimetal redox system, affording the corresponding adducts 4. In contrast, reaction of 2 with Grignard reagents mainly gave one or two electron reduction products, e.g., hydrocoupling dimmer 5 and hydrogenation product 6.
