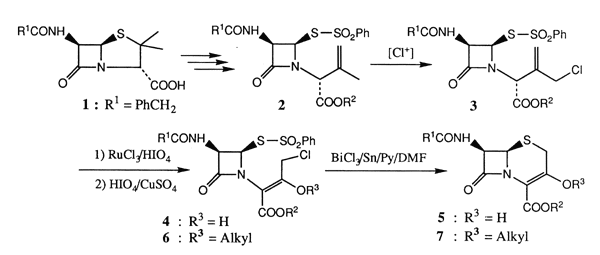
A Facile Access to 3-Hydroxycephems from Penicillin G through Bi/Sn or Ti/Sn Redox-Promoted Cyclization of 4-(Phenylsulfonylthio)azetidinones
Tanaka, H.; Taniguchi, M.; Kameyama, Y.; Monnin, M.; Sasaoka, M.; Shiroi, T.; Nagao, S.; Torii, S. Chem. Lett. 1990, 1867.
Direct conversion of 1-(3-chloro-2-hydroxy-1-p-methoxybenzyloxycarbonyl-1-propenyl)-4-(phenylsulfonylthio)azetidinones derived from penicillin G into 3-hydroxycephems was performed successfully by the action with BiCl3/Sn or TiCl4/Sn bimetal redox couples in DMF containing pyridine.
