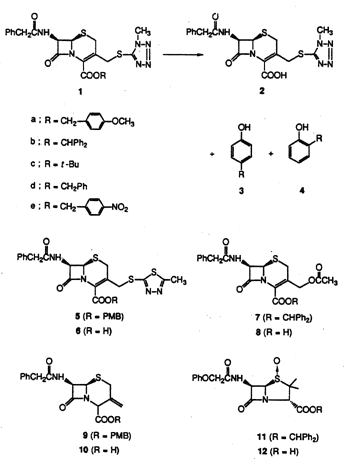
Deprotection of Carboxylic Esters of b-Lactam Homologues. Cleavage of p- Methoxybenzyl, Diphenylmethyl, and t-Butyl Esters Effected by a Phenolic Matrix
Torii, S.; Tanaka, H.; Taniguchi, M.; Kameyama, Y.; Sasaoka, M.; Shiroi, T.; Kikuchi, R.; Kawahara, I.; Shimabayashi, A.; Nagao, S. J. Org. Chem. 1991, 56, 3633.
p-Methoxybenzyl, diphenylmethyl, and t-butyl esters were deprotected by gentle heating in phenol. This method of ester cleavage played an important role in b-lactam synthesis. The mechanism of the reaction is believed to involve a proton relay through a hydrogen-bonded phenolic matrix.
