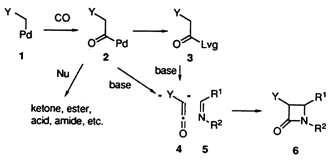
Palladium-Catalyzed Carbonylative [2+2] Cycloaddition for the Stereoselective Sysnthesis of Either cis- or trans-3-Alkenyl-b-Lactams
Tanaka, H.; Hai, A. K. M. A.; Sadakane, M.; Okumoto, H.; Torii, S. J. Org. Chem. 1994, 59, 3040.
A new candidate for the alkenylketene equivalent without use of carboxylic acid and its activated derivatives has been exploited. Palladium-catalyzed carbonylation of allyl phosphates in the presence of imines and teriary amine under CO pressure (30 Kgcm-2) gave stereoselectively either cis- or trans-3-alkenyl-b-lactams in high yields. The stereochemical outcome highly depends on the nature of the imines employed. The carbonylative cycloaddition with the imines conjugated with carbonyl such as ketone and ester stereoselectively produced cis-b-lactams at room temperature, while employment of the other imines nonconjugated with carbonyl groups resulted in the exclusive formation of the trans-b-lactams at 70 íC.
