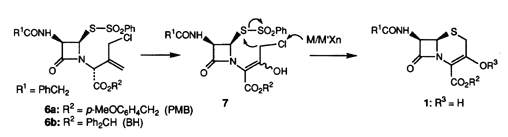
Synthesis of 3-Hydroxycephems from Penicillin G through Cyclization of Chlorinated 4-Phenylsulfonyl-thio-2-azetidinones Promoted by a BiCl3/Sn or TiCl4/Sn Bimetal Redox System
Tanaka, H.; Taniguchi, M.*;
Kameyama, Y.; Monnin, M.; Torii, S.; Sasaoka, M.*; Shiroi, T.*;
Nagao, S.*; Yamada, T.*; Tokumaru, Y.* (*Tokushima Research Laboratories,Otsuka
Chemical Co., Ltd.), Bull. Chem. Soc. Jpn. 1995,
68, 1385.
A novel access to 3-hydroxycephems
was attained from penicillin G via the C=C bond cleavage of 1-(1-alkoxycarbonyl-2-chloromethyl-2-propenyl)-4-phenylsulfonylthio-2-azetidinones
by successive treatment with RuCl3/HIO4 and HIO4/CuSO4 and reductive cyclization of 1-(1-alkoxycarbonyl-3-chloro-2-hydroxy-1-propenyl)-4-phenylsulfonylthio-2-azetidinones
on treatment with a newly devised BiCl3/Sn
or TiCl4/Sn bimetal redox couple in DMF containing
pyridine.
