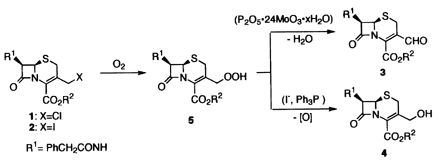
Aerobic Oxidation of 3-Iosomethylcephem to 3-Formylcephem through 3-Hydroperoxymethylcephem
Tanaka, H.; Kikuchi, R.; Torii, S. Bull. Chem. Soc. Jpn. 1996, 69, 229.
Aerobic oxidation of p-methoxybenzyl 3-iodomethyl-8-oxo-7-phenylacetamido-1-aza-5-thiabicyclo[4.2.0]oct-2-ene-2-carboxylate (3-iodomethyl-3-cephem) in N-methyl-2-pyrrolidone in the presence of phosphomolybic acid mainly affroded the corresponding 3-formyl-3-cephem, while similar aerobic oxidation in the presence of potassium iodide gave 3-hydroxymethyl-3-cephem as a major product. 3-Hydroperoxymethyl-3-cephem was isolated as a primary product in the aerobic oxidation, which was subsequently converted to either 3-formyl-3-cephem by dehydration with phosphomolybdic acid or 3-hydroxymethyl-3-cephem by reduction with potassium iodide.
