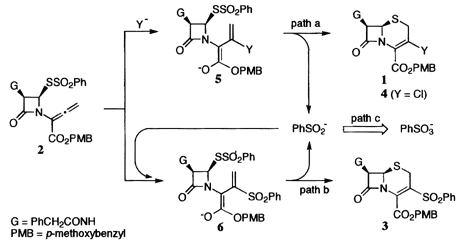
Sythesis of 3-Chloro-D3-cephem-4-carboxylate by Addition/Cyclization of Allenecarboxylate. Copper(II)-Promoted Aerobic Oxidation of Arensulfinic Acids
Tanaka, H.; Kukuchi, R.; Torii, S. Bull. Chem. Soc. Jpn. 1996, 69, 1391.
The selective transfromation of allenecarboxylate derived from penicillin into 3-chloro-D3-cephem-4-carboxylate was successfully achieved by an addition/cyclization reaction with chloride salts in aerobic media containing a copper(II) catalysts, in which copper(II)-catalyzed aerobic oxidation of in situ generated benzenesulfinate ion into less nucleophilic sulfonate ion prior to the nucleophilic addition of the former ion to the allenecarboxylate would completely eliminate the formation of undesired 3-phenylsulfonyl-D3-cephem-4-carboxylate. Under similar aerobic conditions, arenesulfinate salts and arenesulfinic acids were smoothly oxidized to the corresponding sulfonate salts and sulfonic acids, respectively.
