Transformation of Penicillins into 3-Substituted D3-Cephems through Allenecarboxylates
Tanaka, H.; Sumida, S.-i.; Kameyama, Y.; Sorajo, K.; Wada, I.; Torii, S. Bull. Chem. Soc. Jpn. 1996, 69, 3651.
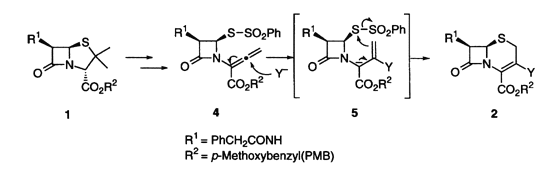
A straightforward synthesis of D3-cephems 2 bearing heteroarom substituents directly attached to C(3)-position was performed successfully by a sequential addition/cyclization reaction of allenecarboxylate 4 derived from penicillin 1. Upon treatment of the allenecarboxylate 4 with morpholine, pyrrolidine, sodium azide, and 5-methyl-1,3,4-thiadiazole-2-thiol in DMF in the presence of calcium chloride, the addition/cyclization reaction proceeded smoothly to afford the corresponding 3-substituted D3-cephems 2a-e, respectively. Reaction of the allenecarboxylate 4 with lithium chloride in NMP (N-methyl-2-pyrrolidone) in the presence of aluminium chloride afforded 3-chloro-D3-cephem 2f, while without aluminium chloride 3-phenylsulfonyl-D3-cephem 2e was mainly obtained. The 3-sulfenyl-D3-cephems 12 were prepared by a similar addtion/cyclization reaction of allencarboxylates 10 with a catalytic amount of thiolates. The sulfenyl moieties attached at the sulfur atom of the C(4)-substituent of 10 were introduced at the C(3)-position of the cephem framework 2.
