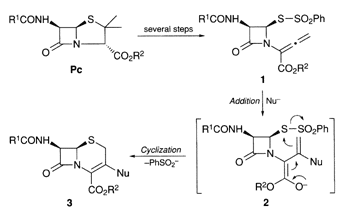
Construction of Cephem Framework via Sequential Reductive 1,2-Elimination-Hydride Addition in a Tributyltin Hydride -Copper(I) Chloride-NMP System: Synthesis of 3-Norcephalosporin
Tanaka, H; Yamaguchi, Y.; Sumida, S.-i.; Torii, S. J. Chem. Soc., Chem. Commun. 1996, 2705.
A sequential reductive 1,2-elimination and hydride addition of 3,4-disubstituted butenoates derived from penicillin was successfully performed by the aid of a combination of tributyltin hydride and copper(I) chloride in N-methyl-2-pyrrolidinone (NMP) to afford 3-norcephalosporin.
