Oxidative Addition of Aryl Halides to Transient Anionics-Aryl-Palladium(0) Intermediates---Application to Palladium-Catalyzed Reductive Coupling of Aryl Halides
Amatore, C.; CarrŽ, E.; Jutand, A.; Tanaka, H.; Ren, Q.; Torii, S. Chem. Eur. J. 1996, 2, 957.
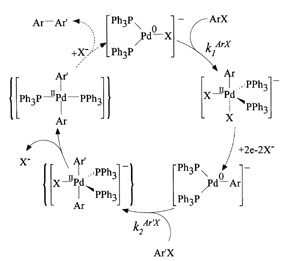
The rates and mechanism of the reactions of a series of aryl-ligated, anionic palladium(0) complexes Ar-Pd0-(PPh3)2 with para-substituted iodobenzenes were investigated by means of transient electrochemistry. The reaction was found to be first order in each reactant and to proceed similarly to oxidative addition of aryl halides to the halide-ligated species X-Pd0(PPh3)2-, although much faster and less sensitive to electronic factors. Owing to the short lifetime (t1/2Ĺ1-5 ms) of product of this reaction, it could not be characterized in detail. However, based on kinetic results, this transient species is thought to be an anionic pentacoordinated bisarylpallasium(II) complex, which undergoes rapid loss of halide ligand to yield, most probably, a bisarylpalladium(II) neutral species. Based on the study of this reaction and on previously reported results, we propose a mechanism for the palladium-catalyzed homocoupling of aryl halides consisting of a catalytic cycle initiated by oxidative addition of an aryl halide to a zerovalent tris-ligated palladium center. Two-electron reduction of the pentacooedinated arylpalladium(II) anionic species thus formed gives a tris-ligated anionic arylpalladium(0) center, which undergoes oxidative addition with a second aryl halide molecule to eventually lead to a bisarylpalladium(II) neutral species. Reductive elimination of a bisaryl molecule from this center closes the catalytic cycle by regenerating the initial zerovalent palladium complex. The application of this sequence to the catalytic heterocoupling of aryl halides is discussed, and it is concluded, on the basis of Hammett correlations, that statistical yields should be observed, in agreement with the results obtained for preparative reactions in DMF.
