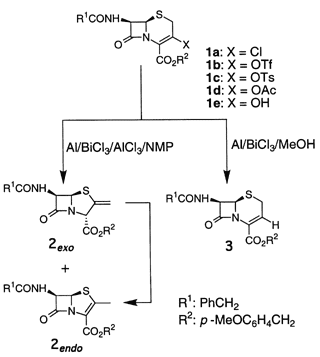
Conversion of Cephem into Penam/Penem through Sequential Ring-Opening/Recyclization in an Al/BiCl3/AlCl3 System
Tanaka, H.; Tokumaru, Y.; Kameyama, Y.; Torii, S. Chem. Lett. 1997, 1221.
A sequential reductive ring-opening/recyclization reaction of 3-substituted D3-cephems into 2-exo-methylenepenams and/or 2-methylpenems was successfully performed by treatment with Al/BiCl3 in NMP (N-methyl-2-pyrrolidinone). When the reaction was carried out in methanol, 3-norcephalosporin was formed as a major product.
