Synthesis of Optically Active 1,3-Dienes from Alkenylstannanes by a Combination of Electro-Oxidation and Copper-Mediated Homo-Coupling Reaction
Itoh, T.*; Emoto, S.*; Ohara, H.*; Tanaka, H.; Torii, S (*Faculty of Education), Electrochim. Acta 1997, 42, 2133.
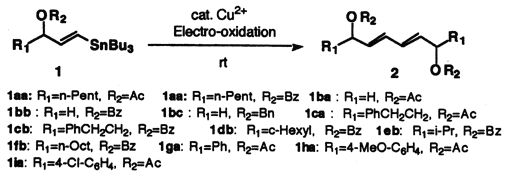
A combination of electro-oxidation and copper(II) chloride mediated homocoupling ofg-acetoxyvinylstannanes afforded 1,3-dienes. The stereoselectivity strongly depended on the solvent system. No isomerization occurred when the reactions were carried out in DMF(0.01M). The facile synthesis of dibenzoate of (6R,11R)-(E,E)-hexadeca-7,9-diene-6,11-diol was thus accomplished from (R)-1-tributylstannyl-1-(E)-octene-3-ol, which was prepared through a lipase-catalyzed reaction in an optically active form.
