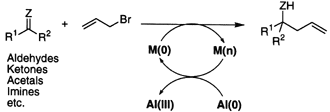
Barbier-type Allylation of Carbonyl Derivatives by Use of Aluminium as an Electron Pool. Double Allylation of Carboxylic Esters
Tanaka, H.; Nagahata, S.; Watanabe, H.; Zhao, J.; Kuroboshi, M.; Torii, S. Inorg. Chim. Acta 1999, 296, 204.
Double allylation of carboxylic esters with allyl bromide was performed successfully by the action of Al metal and a catalytic amt. of Pb(II) bromide in THF. E.g., PhCH2CO2Me reacted with allyl bromide, Al and PbBr2 catalyst in THF at room temp. to give 98% yield of PhCH2CHOH(CH2CH:CH2)2. The proper choice of solvent is essential for the reaction; thus, among the examined solvents, ethers, e.g., THF, 1,2-dimethoxyethane (DME), and Et2O, could be successfully used for the double allylation but with DMF, aq. MeOH, and aq. THF, no appreciable reaction occurred. Allylation of benzaldehyde di-Me acetal and N-benzylimine in THF under the same conditions took place smoothly to afford the corresponding allylation products. In a similar manner, allylation of benzonitrile was also performed to afford the doubly allylated benzylamine.
