Electrogeneration of Triphenyltin Radical, Anion and Cation. Electrochemical Initiation of Tin Hydride-Promoted Radical Chain Reactions.
Tanaka, H.; Ogawa, H.; Suga, H.; Torii, S.; Jutand, A.*; Aziz, S.*; Suarez , A. G.*; Amatore, C. * (*Ecole Normale Superieure), J. Org. Chem. 1996, 61, 9402.
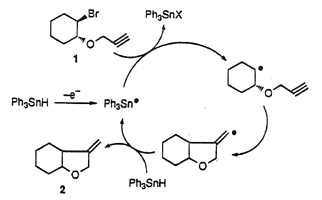
In our research of precursors of tin radicals able to initiate radical chain reactions under mild conditions, a series of triphenyltin derivatives Ph3SnY (Y = H, I, Cl, OTf, OCHO, SnPh3, SPh ) is investigated by cyclic voltammetry. The results show that the tin radical Ph3SnĄ is only produced from two compounds: by a one electron oxidation of Ph3SnH or by a one electron reduction of Ph3SnI. Therefore electrooxidation of Ph3SnH generates Ph3SnĄ which is able to initiate cyclization of haloalkynes. Reduction or oxidation of the other derivatives affords respectively the anion Ph3Sn- or the cation Ph3Sn+ because they are generated at potentials where the radical Ph3SnĄ is either reduced or oxidized.
