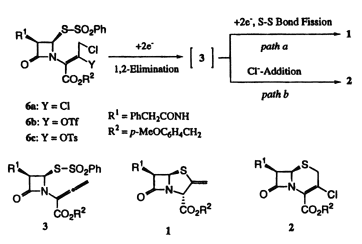
Synthesis of 2-exo-Methylenepenam and 3-Chloro-D3-cephem through a Sequential Reductive 1,2-Elimination/S-S Bond Fission or Chloride Ion-Addition/Cyclization of 3,4-Disubstituted 2-Butenoates in Metal Combinations
Tanaka, H.; Sumida, S. -i.; Nishioka, Y.; Kobayashi, N.; Tokumaru, Y.; Kameyama, Y.; Torii, S. J. Org. Chem. 1997, 62, 3610.
Synthesis of 2-exo-methylenepenam 1 through a sequential reductive 1,2-elimination/S-S bond fission/cyclization of 6 was performed by treatment with a PbBr2/Al (or a BiCl3/Al) combination in DMF, while that of 3-chloro-D3-cephem 2 through reductive 1,2-elimination/chloride ion-addition/cyclization was attained by use of an AlCl3/Al combination in N-methylpyrrolidone (NMP). The selective transformation of 6 to the allenecarboxylate 3 was also achieved by treatment with an AlBr3/Al combination in NMP. Cyclic voltammograms of 3,4-disubstituted 2-[2-oxo-3-(phenylacetamido)-4-(phenylsulfonylthio)azetidin-1-yl]-2-butenoates (6) exhibit two irreversible reduction peaks responsible for reductive 1,2-elimination of 3,4-disubstituted 2-butenoate moiety (at less negative potential) and for reductive S-S bond fission of phenylsulfonylthio moiety, suggesting that the reductive 1,2-elimination of 6 leading to allenecarboxylate 3 would occur prior to the reductive S-S bond cleavage.
