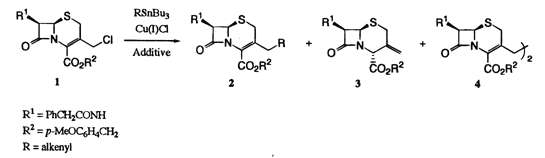
Synthesis of 3-Alkenyl- and 3-Arylalkyl-D3-cephems by Use of Terpyridine or Bipyridine-Ligated Organocopper Species
Tanaka, H.; Sumida, S.-i.; Torii,S. J. Org. Chem. 1997, 62, 3782.
Reaction of 3-chloromethyl-D3-cephem 1 with organotins in N-methyl-2-pyrrolidinone (NMP) in the presence of copper(I) chloride and terpyridine afforded 3-alkenyl-D3-cephems 2a-d. Homo-coupling reaction of the 3-chloromethyl-D3-cephem 1 was performed by treatment with copper powder and bipyridine in NMP to afford the corresponding dimer 4. Similarly, cross-coupling reaction of 1 with allyl and benzyl bromides proceeded to give 3-(3-butenyl)-D3-cephem 2c and 3-arylalkyl-D3-cephems 2g-i, respectively. The presence of terpyridine or bipyridine is indispensable in every reaction; otherwise, no detectable amounts of the products 2 or 4 were obtained. Terpyridine and bipyridine seem to coordinatively stabilize the organocopper species, generated either by transmetallation of organotins with copper(I) chloride or by reaction of 1 and/or allyl and benzyl bromides with copper powder, to promote the desired reactions.
