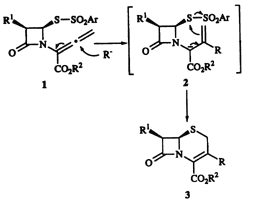
Facile Access to 3-Allyl- and 3-Benzyl-D3-cephems through Reductive Addition-Cyclization of Allenecarboxylate with Allylic and Benzylic Halides in an [NiCl2(bipy)]/PbBr2/Al Redox System
Tanaka, H.; Sumida, S.-i.; Sorajo, K.; Kameyama, Y.; Torii,S. J. Chem. Soc., Perkin Trans. 1. 1997, 637
A sequential reductive addition/cyclization reaction of allenecarboxylate 1, derived from penicillin G, with allylic and benzylic halides was successfully achieved by the aid of a three metal redox system consisting of aluminium metal (2.5 molar amounts) and catalytic amount of [NiCl2(bipy)] (0.1 molar amounts) and PbBr2 (0.05 molar amounts) in N-methyl-2-pyrrolidone (NMP) to afford the corresponding 3-allyl- and 3-benzyl-D3-cephems 3a-i in 20-85% yields. The reactions of 1 with vinyl, propargyl, and phenyl halides in the same three metal redox system resulted in the recovery of 1 and /or partial formation of 2-exo-methylenepenam 4. A similar electroreductive addition/cyclization of 1 with allyl bromide was performed by passage of 3.2 F/mol electricity in an [NiCl2(bipy)]/PbBr2/NMP/(Pt cathode)-(Al anode) system.
