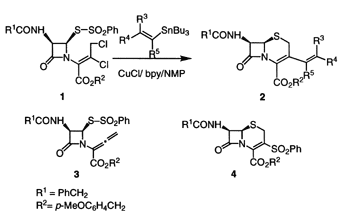
Synthesis of 3-Alkenyl-D3-cephems via Sequential Reductive 1,2-Elimination/Addition/Cyclization in an Alkenyltin/Copper(I) Chloride/bpy Systems.
Tanaka, H.; Tokumaru, Y.; Torii, S. Synlett, 1999, 774.
A sequential reductive 1,2-elimination-addition-cyclization of 3,4-disubstituted
butenoates derived from penicillin is performed successfully by treatment
with alkenyltin, CuCl, and 2,2'-bipyridine in N-methyl-2-pyrrolidinone
to afford 3-alkenyl-3,4-didehydrocephems.
