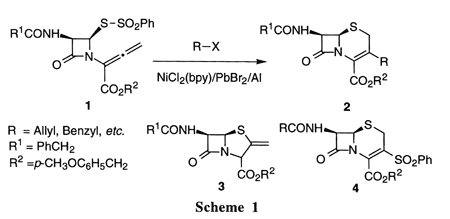
Synthesis of 3-allyl- and 3-benzyl-D3-cephems through sequential reductive
1,2-elimination/addition/cyclization of 3,4-disubstituted 2-butenoates
in allyl and benzyl halides/Mn/NiCl2/AlCl3/NMP systems.
Tanaka, Hideo; Tokumaru, Yoshihisa; Torii, Sigeru.
Heterocycles (2000), 52(2), 633-642.
One-pot synthesis of 3-allyl- and 3-benzyl-D3-cephems through a sequential
reductive 1,2-elimination/addn./cyclization of 3,4-disubstituted 2-[2-oxo-3-phenylacetamido-4-(phenylsulfonylthio)azetidin-1-yl]-2-butenoates
was successfully performed by treatment with allyl and benzyl halides in
an Mn/NiCl2(bpy)/AlCl3/N-methyl-2-pyrrolidinone (NMP) system. E.g., tosylate
I (R = SO2Ph, R1 = 4-methoxybenzyl) was reacted with allyl bromide in the
presence of Mn powder, AlCl3, and (2,2'-bipyridine)dichloronickel in NMP
at room temperature under argon for 20 min to form cephem II (R1 = 4-methoxybenzyl)
as the major product in 80% yield.
