Reinvestigation of the Biomimetic Cyclization of 3,5-Diketoesters: Application to the Total Synthesis of Cyercene A, an α-Methoxy-γ-Pyrone-Containing Polypropionate
Kai Onda, Ichiro Hayakawa, Akira Sakakura*
Synlett2017, 28 (13), 1596–1600. (Published online: Apr 26, 2017)
DOI: 10.1055/s-0036-1588795 
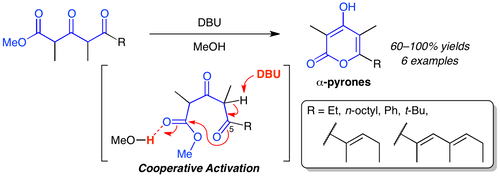
The biomimetic cyclization of 3,5-diketoesters was reinvestigated for the synthesis of α-methoxy-γ-pyrones. 3,5-Diketoesters were selectively synthesized via the aldol reaction of commercially available methyl 2-methyl-3-oxopentanoate with an aldehyde followed by the oxidation with AZADOL® and PhI(OAc)2. The DBU-promoted intramolecular transesterification of 3,5-diketoesters showed excellent reactivity in MeOH, to give the corresponding γ-hydroxy-α-pyrones in high yields under mild reaction conditions. Based on the present cyclization scheme, the total synthesis of cyercene A was achieved.