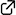2025年度
Development of a Vinylated Cyclic Allene: A Fleeting Strained Diene for the Diels–Alder Reaction
Haruki Mizoguchi*, Takumi Obata, Taiki Hirai, Manaka Komatsu, Akira Sakakura*
Angew. Chem. Int. Ed. 2025, 64, e202510319
DOI: 10.1002/anie.202510319
A strain-promoted Diels–Alder reaction of the vinylated cyclic allene, a highly reactive strained all-carbon diene, was developed. Activation of the Kobayashi-type precursor with fluoride ion generated the vinyl cyclic allene, which was trapped by dienophiles to produce decalin derivatives under mild conditions. This method would provide an efficient route to synthesize natural product-relevant multicyclic skeletons in a convergent fashion.
2024年度
Synthesis of an Angular Triquinane Structure Based on a Stereoselective Decarboxylative Giese Reaction at an Angular Position in a Diquinane Skeleton
Naochika Matsumaru, Takumu Nishitani, Haruki Mizoguchi*, Akira Sakakura*
Tetrahedron 2025, 178, 134612.
DOI: 10.1016/j.tet.2025.134612
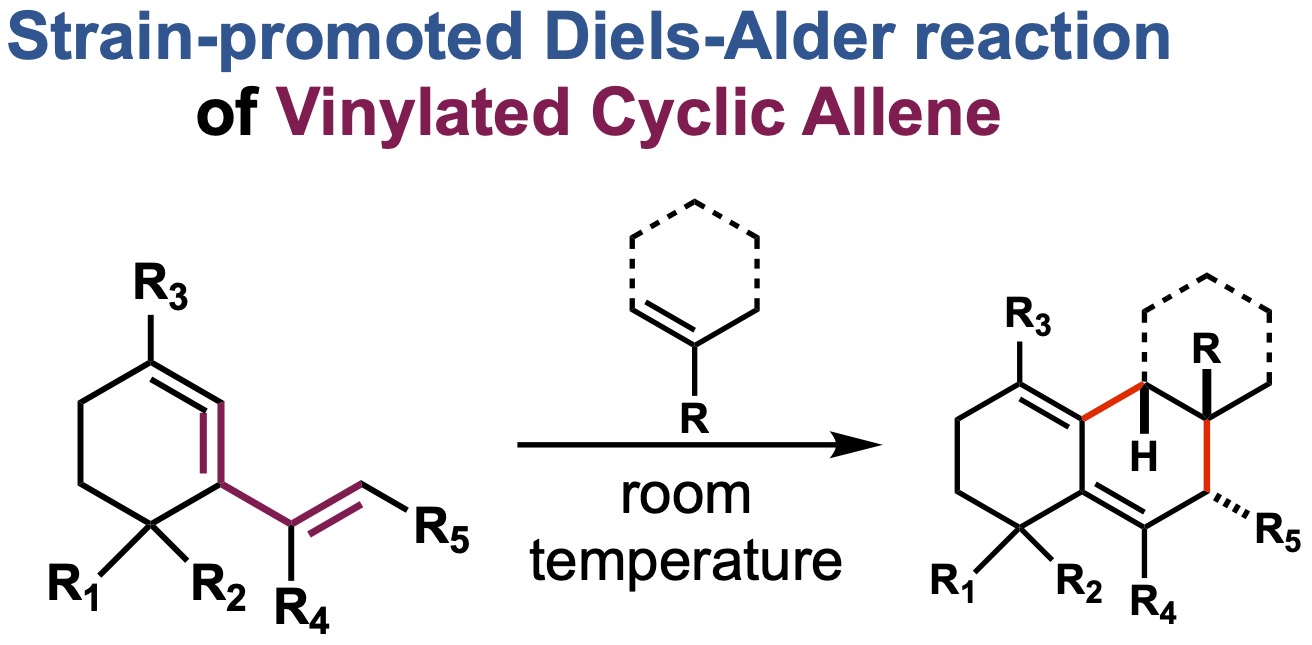
A chemo-, regio-, and stereo-selective method for constructing the angular triquinane core-skeleton was developed based on a radical carbon-carbon bond formation at a diquinane ring-junction as a key process. With the use of a photoredox catalyst under blue LED irradiation, the decarboxylative Giese reaction of the diquinane core with unsaturated carbonyl compounds proceeds stereoselectively to construct a quaternary carbon center at the angular position. Subsequent aldol cyclization exploiting the carbonyl group installed to a diquinane core resulted in an expeditious synthesis of the angular triquinane skeleton.
Rapid construction of a diterpene-inspired tetracyclic skeleton bearing bicyclo[3.2.1]octane rings based on desymmetrization of 1,3-diketones
Hidetoshi Kamada, Haruki Mizoguchi*, Akira Sakakura*
Tetrahedron Lett. 2025, 157, 155470
DOI: 10.1016/j.tetlet.2025.155470
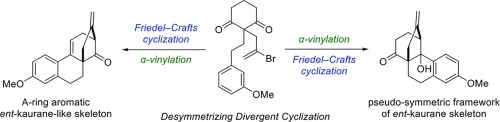
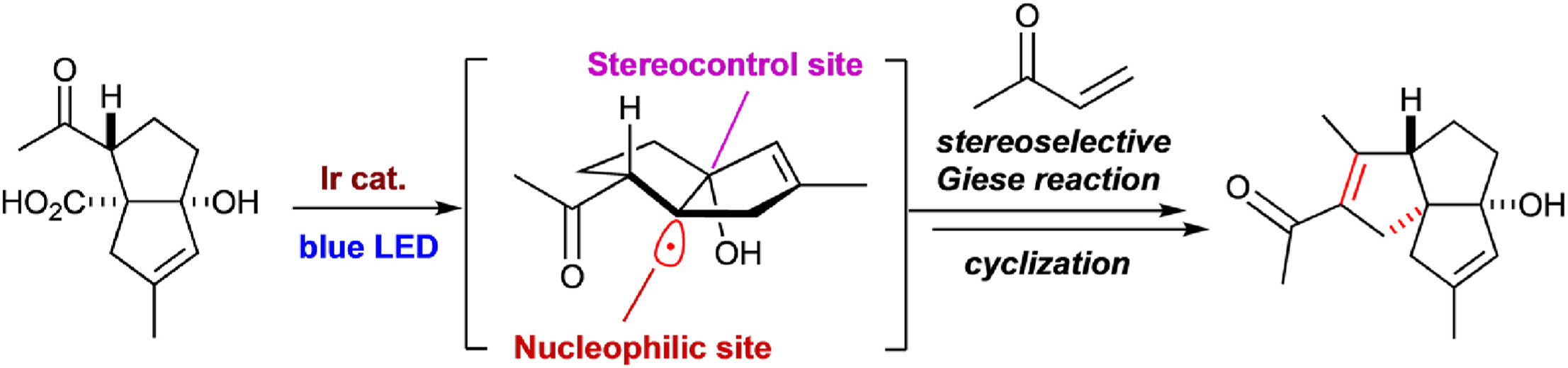
A synthetic methodology for constructing ent-kaurane-like tetracyclic skeletons possessing a bicyclo[3.2.1]octane moiety has been developed by employing a desymmetrizing sequential cyclization strategy. Using a symmetric diketone as a key branching point, the sequence of desymmetric Friedel–Crafts cyclization and Pd-catalyzed intramolecular α-vinylation of ketone allowed us to construct the ent-kaurane-like tetracyclic skeleton from 1,3-cyclohexanedione in four steps. In contrast, reversing the order of the cyclization steps yielded a pseudo-symmetric tetracyclic skeleton, which is also reminiscent of the kaurane framework.
2023年度
Synthesis and biological evaluation of coprinoferrin, an acylated tripeptide hydroxamate siderophore
Ichiro Hayakawa*, Tomoki Isogai, Jun Takanishi, Shihori Asai, Chika Ando, Tomohiro Tsutsumi, Kenji Watanabe*, Akira Sakakura* and
Yuta Tsunematsu*
Org. Biomol. Chem. 2024, 22, 831–837
DOI: 10.1039/D3OB01850D
Coprinoferrin (CPF), originally isolated from a genetically engineered strain (ΔlaeA) of the mushroom fungus Coprinopsis cinerea, is an acylated tripeptide hydroxamate consisting of tandem aligned N5-hexa- noyl-N5-hydroxy-L-ornithine with modifications of N-acetyl and C-carboxamide. These unique chemical properties make CPF an iron(III) binder (siderophore), which helps in iron acquisition from the environment and promotes hyphal growth as well as fruiting body formation in C. cinerea. However, CPF’s detailed mode of action remains enigmatic. In this study, we have accomplished the synthesis of CPF from N-Boc-L-glutamic acid 5-benzyl ester. The physicochemical characteristics, spectroscopic features, and biological activity observed in the synthetic CPF closely match those of natural CPF. This alignment pro- vides unequivocal confirmation of the proposed chemical structure, facilitating a deeper understanding of its physiological role in nature, particularly in fruiting body formation.
Visible-Light-Photoexcited Palladium-Catalyzed Silylmethylation of Benzyl Alcohol Derivatives
Haruki Mizoguchi*,Ryuji Yoshida, Haruka Ikeda, Akira Sakakura*
Synlett 2023, eFirst (Special Issue Dedicated to Prof. Hisashi Yamamoto)
DOI: 10.1055/s-0042-1752736
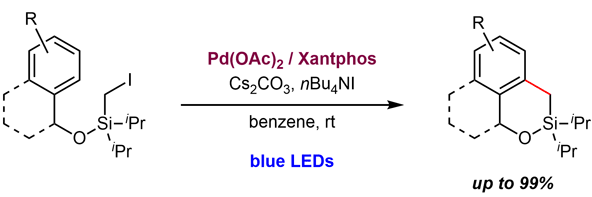
An intramolecular C–H silylmethylation of benzylic alcohol derivatives catalyzed by a visible-light-photoexcited palladium complex was developed. Irradiation of the palladium complex with blue LEDs resulted in efficient activation of a C–I bond, and subsequent intramolecular radical arylation afforded siloxacycle products. Selective protonation or oxidation of the C–Si bond of the cyclized materials afforded ortho-methylated and ortho-hydroxymethylated derivatives of the benzylic alcohol derivatives.
2022年度
Annulative coupling of vinylboronic esters: aryne-triggered 1,2-metallate rearrangement
Haruki Mizoguchi*, Hidetoshi Kamada, Kazuki Morimoto, Ryuji Yoshida, Akira Sakakura*
Chem. Sci. 2022, 13, 9580–9585.
DOI: /10.1039/D2SC02623F
A stereoselective annulative coupling of a vinylboronic ester ate-complex with arynes producing cyclic borinic esters has been developed. An annulation reaction that proceeded through the formation of two C–C bonds and a C–B bond was realized by exploiting a 1,2-metallate rearrangement of boronate triggered by the addition of a vinyl group to the strained triple bond of an aryne. The generated aryl anion would then cyclize to a boron atom to complete the annulation cascade. The annulated borinic ester could be converted to boronic acids and their derivatives by oxidation, halogenation, and cross-coupling. Particularly, halogenation and Suzuki–Miyaura coupling proceeded in a site-selective fashion and produced highly substituted alkylboronic acid derivatives.
Enantioselective construction of β-hydroxy-α,α-disubstituted α-amino acid derivatives via direct aldol reaction of α-imino esters
Yuya Araki, Masato Hanada, Yoshiko Iguchi, Haruki Mizoguchi, Akira Sakakura*
Tetrahedron 2022, 110, 132695.
DOI: 10.1016/j.tet.2022.132695
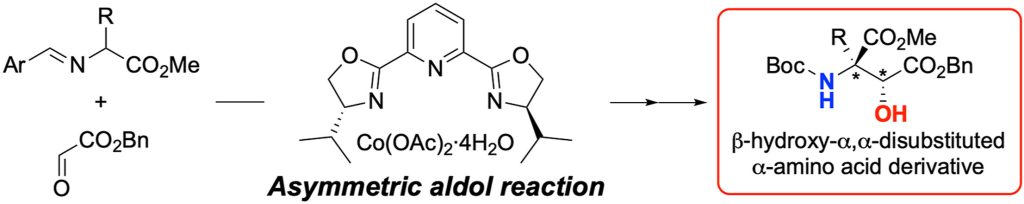
The β-hydroxy-α,α-disubstituted α-amino acid is a valuable structural motif for research in the field of bioorganic chemistry and in the development of peptide drugs. This report describes the enantioselective direct-aldol reaction of α-imino esters with glyoxylate esters. We discovered that a catalytic amount of Co(OAc)2-pybox complex catalyzed the aldol reaction of salicylaldehyde-derived α-imino esters with benzyl glyoxylate in good yield and enantioselectivity. In addition, hydrolysis of the imine moiety of the aldol products followed by Boc protection of the resultant amino group gave the N-Boc-protected amino acid derivatives.
2021年度
Toward the Synthesis of Paspaline-Type Indole-Terpenes: Stereoselective Construction of Core Scaffold with Contiguous Asymmetric Quaternary Carbon Centers
Ichiro Hayakawa*, Naochika Matsumaru, and Akira Sakakura*
J. Org. Chem. 2021, ASAP (Published online: July 7, 2021)
DOI: 10.1021/acs.joc.1c01193
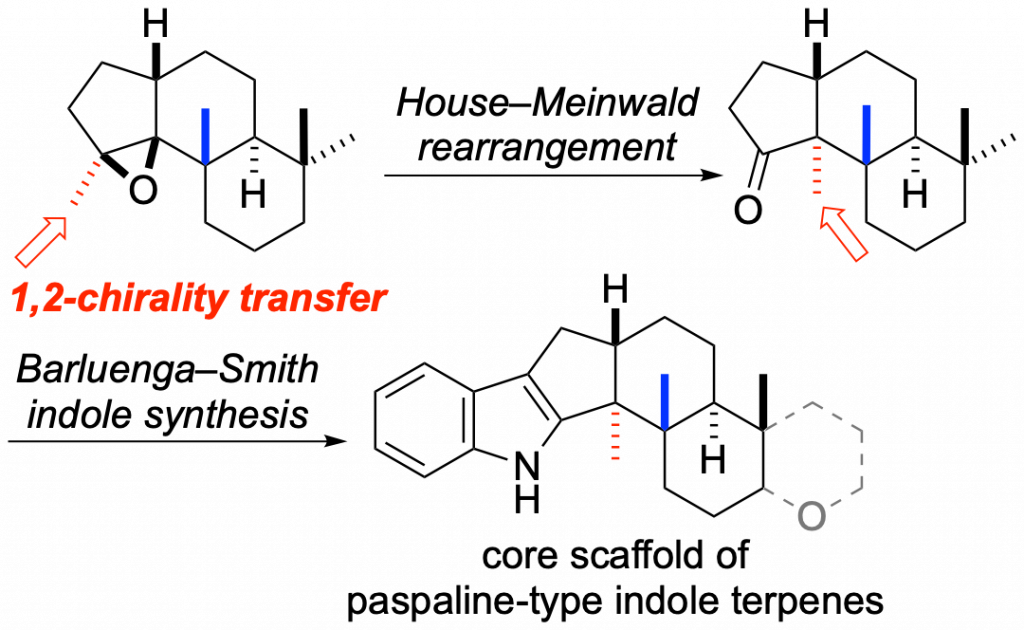
The core scaffold of paspaline-type indole-terpenes was synthesized by using the House–Meinwald rearrangement as a key step. Rearrangement of the epoxide methyl group in the precursor with methylaluminum bis(4-bromo-2,6-di- tert-butylphenoxide) as a Lewis acid proceeded smoothly to construct contiguous asymmetric quaternary carbon centers by a 1,2-chirality transfer.
Strain-Release Difunctionalization of C–C σ- and π-bonds of an Organoboron Ate-Complex through 1,2-Metallate Rearrangement
Haruki Mizoguchi,* Akira Sakakura*
Chem. Lett. 2021, 50 (4), 792–799. (Published online: January 13, 2021)
DOI: 10.1246/cl.200926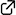
This highlight review describes the recent development of an electrophile-triggered 1,2-metallate rearrangement of organoboronic ester ate-complex, which proceeds through 1,2-difunctionalization of carbon–carbon σ- and π-bonds, using strain energy as a driving force. Coupling reactions of small ring carbocyclic boronic esters, such as cyclopropyl-, bicyclo[1.1.0]butyl-, and cyclopropenyl-boronic ester, are summarized along with the proposed reaction mechanisms and representative examples.
2020年度
Synthesis of functionalized cyclopropylboronic esters based on a 1,2-metallate rearrangement of cyclopropenylboronate
Haruki Mizoguchi,* Masaya Seriua, Akira Sakakura*
Chem. Commun. 2020, 56 (99), 15545–15548. (Published online: November 18, 2020)
DOI: 10.1039/d0cc07134j 

A procedure converting tribromocyclopropane to densely functionalized β-selenocyclopropylboronic ester using the 1,2-metallate rearrangement of a boron ate-complex has been developed. Treatment of an in situ-generated cyclopropenylboronic ester ate-complex with phenylselenenyl chloride triggered stereospecific rearrangement to produce functionalized cyclopropanes. DFT calculations for 1,2-metallate rearrangement suggested that the reaction proceeds through a seleniranium intermediate.
Kinetic Resolution of α-Nitrolactones by Catalytic Asymmetric Hydrolysis or Ester–Amide Exchange Reaction
Ryota Nakao, Yudai Fujii, Ichiro Hayakawa, Haruki Mizoguchi, Akira Sakakura*
Synlett 2020, 31 (20), 2018–2022. (Published online: October 8, 2020)
DOI: 10.1055/s-0040-1707303 
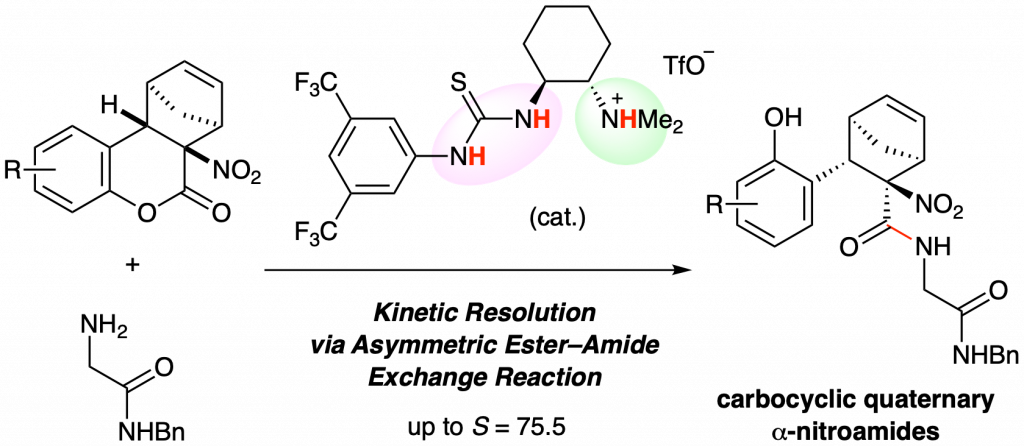
C1-Symmetric chiral ammonium salt catalysts induced a kinetic resolution of racemic α-nitrolactones through an asymmetric ester–amide exchange reaction. The corresponding amides were obtained with high enantioselectivities and high S (= kfast/ kslow) values. This reaction system is a useful approach for obtaining carbocyclic quaternary α-nitroamides as chiral building blocks.
Enantioselective Diels–Alder Reaction of 3-Nitrocoumarins Promoted by Chiral Organoammonium Salt Catalysts
Yudai Fujii, Ryota Nakao, Saki Sugihara, Keita Fujita, Yuya Araki, Takayuki Kudoh, Ichiro Hayakawa, Haruki Mizoguchi, Akira Sakakura*
Synlett 2020, 31 (20), 2013–2017. (Published online: October 7, 2020)
DOI: 10.1055/s-0040-1707302 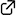
An enantioselective Diels–Alder reaction of 3-nitrocoumarins has been developed. A tryptophan-derived C1-symmetric organoammonium thiourea catalyst promoted the reaction of 3-nitrocoumarins with Danishefsky’s diene to give the corresponding adducts with good enantioselectivity (up to 94% ee). One of the resulting adducts was converted into a chiral carbocyclic quaternary β-amino alcohol.
▶︎ Front Cover

2019年度
Structure Optimization of Gatastatin for the Development of γ-Tubulin-Specific Inhibitor
Kana Shintani, Haruna Ebisu, Minagi Mukaiyama, Taisei Hatanaka, Takumi Chinen, Daisuke Takao, Yoko Nagumo, Akira Sakakura, Ichiro Hayakawa,* Takeo Usui*
ACS Med. Chem. Lett. 2020, 11 (6), 1125–1129. (Published online: March 30, 2020)
DOI: 10.1021/acsmedchemlett.9b00526 
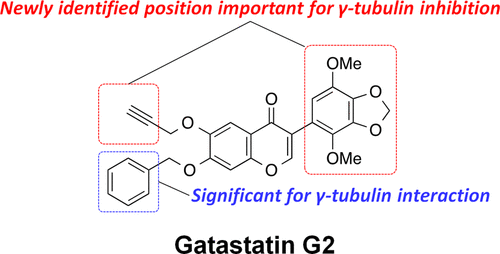
Gatastatin (O7-benzyl glaziovianin A) is a γ-tubulin-specific inhibitor that is used to investigate γ-tubulin function in cells. We have previously reported that the unsubstituted phenyl ring of the O7-benzyl group in gatastatin is important for γ-tubulin inhibition. To obtain further structural information regarding γ-tubulin inhibition, we synthesized several gatastatin derivatives containing a fixed O7-benzyl moiety. Modifications of the B-ring resulted in drastic decrease in cytotoxicity, abnormal spindle formation activity, and inhibition of microtubule (MT) nucleation. In contrast, various O6-alkylated gatastatin derivatives showed potent cytotoxicity, induced abnormal spindle formation, and inhibited MT nucleation. We had previously reported that O6-benzyl glaziovianin A is a potent α/β-tubulin inhibitor; thus, these new results suggest that the O6-position restricts affinity for α/β- and γ-tubulin. Considering that an O7-benzyl group increases specificity for γ-tubulin, more potent and specific γ-tubulin inhibitors can be generated through O6-modifications of gatastatin.
Formal Total Synthesis of Manzacidin B via Sequential Diastereodivergent Henry Reaction
Yuya Araki, Natsumi Miyoshi, Kazuki Morimoto, Takayuki Kudoh, Haruki Mizoguchi, Akira Sakakura*
J. Org. Chem. 2020, 85 (2), 798–805. (Published online: December 18, 2019)
DOI: 10.1021/acs.joc.9b02811

A formal total synthesis of manzacidin B is described. β,β-Disubstituted γ-hydroxy-β-aminoalcohol, the key structure of manzacidin B, is stereoselectively constructed via sequential Henry reactions. By taking advantage of noncovalent interactions, such as intramolecular hydrogen bonding and chelation, we could diastereodivergently control the stereoselectivity of the Henry reaction.
Toward the Synthesis of SB-203207: Construction of Four Contiguous Nitrogen-Containing Stereogenic Centers
Ichiro Hayakawa,* Anna Nagayasu, Akira Sakakura*
J. Org. Chem. 2019, 84 (23), 15614–15623. (Published online: November 8, 2019)
DOI: 10.1021/acs.joc.9b02627
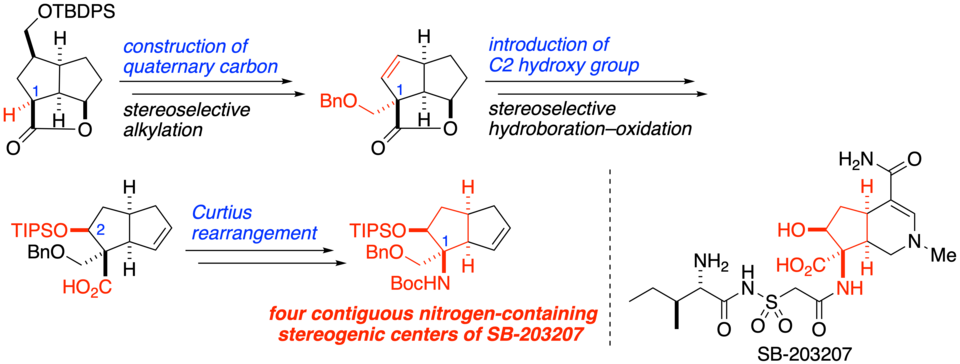
SB-203207 is an altemicidin-type alkaloid that potently inhibits isoleucyl tRNA synthetase activity. Its main structural feature is a hexahydro-6-azaindene framework containing a unique β-hydroxy α,α-disubstituted α-amino acid moiety on the cyclopentane portion. Herein we have established a method for constructing the four contiguous nitrogen-containing stereogenic centers of SB-203207 by using as key steps the stereoselective alkylation of bowl-shaped tricyclic lactone to construct quaternary carbon at C1, the stereoselective hydroboration–oxidation reaction to install the C2 hydroxy group, and the Curtius rearrangement to introduce a nitrogen atom onto the C1 quaternary carbon.
Toward the Synthesis of Yuzurimine-type Alkaloids: Stereoselective Construction of the Heterocyclic Portions of Deoxyyuzurimine and Macrodaphnine
Ichiro Hayakawa,* Ryosuke Nagatani, Masaki Ikeda, Dong-eun Yoo, Keita Saito, Hideo Kigoshi, and Akira Sakakura*
Org. Lett. 2019, 21 (16), 6337–6341. (Published online: July 30, 2019)
DOI: 10.1021/acs.orglett.9b02232 

The heterocyclic portions of yuzurimine-type alkaloids, such as deoxyyuzurimine and macrodaphnine, were synthesized by using a stereoselective hydroboration−oxidation reaction to install the C20 methyl group, the intramolecular Mitsunobu reaction to construct the E-ring portion, and the intramolecular SN2 reaction to construct the F-ring portion as key steps.
Enantioselective 1,3-Dipolar Cycloaddition Reaction of Nitrones with α-(Acyloxy)acroleins Catalyzed by Dipeptide-Derived Chiral Tri- or Diammonium Salts
Chihiro Kidou, Haruki Mizoguchi, Tatsuo Nehira, Akira Sakakura*
Synlett 2019, 30 (15), 1835–1839. (Published online: July 30, 2019)
DOI: 10.1055/s-0039-1690133 
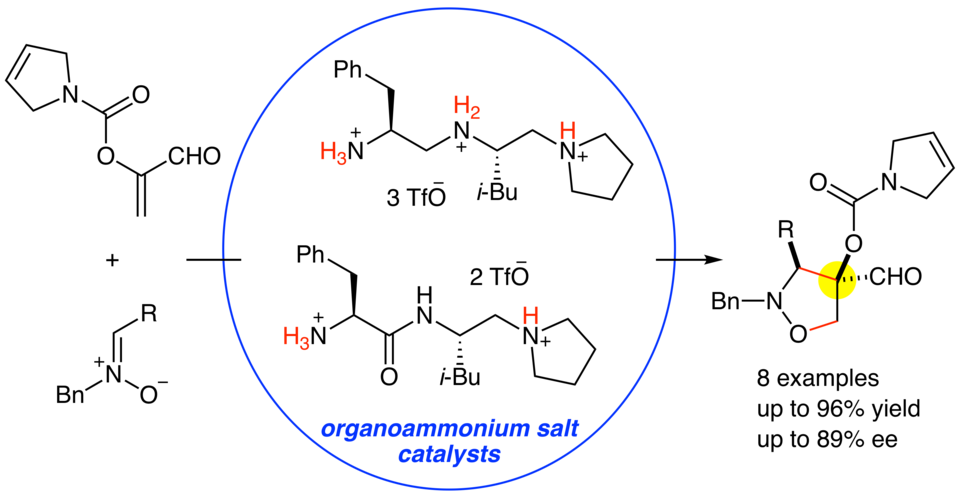
Organoammonium salts of dipeptide-derived chiral triamines or diamines with TfOH catalyzed the enantioselective 1,3-dipolar cycloaddition reactions of α-acyloxyacroleins with nitrones to give the corresponding adducts in good yields (up to 96%) and with high diastereo- and enantioselectivities (up to 89% ee). Although α-( p-methoxybenzoyloxy)acrolein is rather unstable under the reaction conditions, α-(3-pyrroline-1-carbonyloxy)acrolein is stable enough to be smoothly converted into the corresponding adducts with the aid of the chiral organoammonium salt catalysts.
2018年度
Thioureas as Highly Active Catalysts for Biomimetic Bromocyclization of Geranyl Derivatives
Miyuki Terazaki, Kei-ichi Shiomoto, Haruki Mizoguchi, Akira Sakakura*
Org. Lett. 2019, 21 (7), 2073–2076. (Published online: March 12, 2019)
DOI: 10.1021/acs.orglett.9b00352 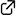
Thioureas bearing electron-deficient aryl groups show high catalytic activity in the biomimetic bromocyclization of geranyl derivatives. The reaction of geranyl derivatives with N-bromosuccinimide (NBS) proceeds rapidly in CH2Cl2 to give the corresponding bromocyclization products in high yields as a ca. 1:1 mixture of endo- and exo-isomers. The reactivity of geranyl derivatives highly depends on the terminal substituent: electron-donating substituents increase the reactivity, while electron-withdrawing substituents decrease the reactivity.
Catalytic Enantioselective Hosomi–Sakurai Reaction of α-Ketoesters Promoted by Chiral Copper(II) Complexes
Yutaro Niwa, Mayu Miyake, Ichiro Hayakawa, Akira Sakakura*
Chem. Commun. 2019, 55 (27), 3923–3926. (Published online: March 5, 2019)
DOI: 10.1039/C9CC01159E 
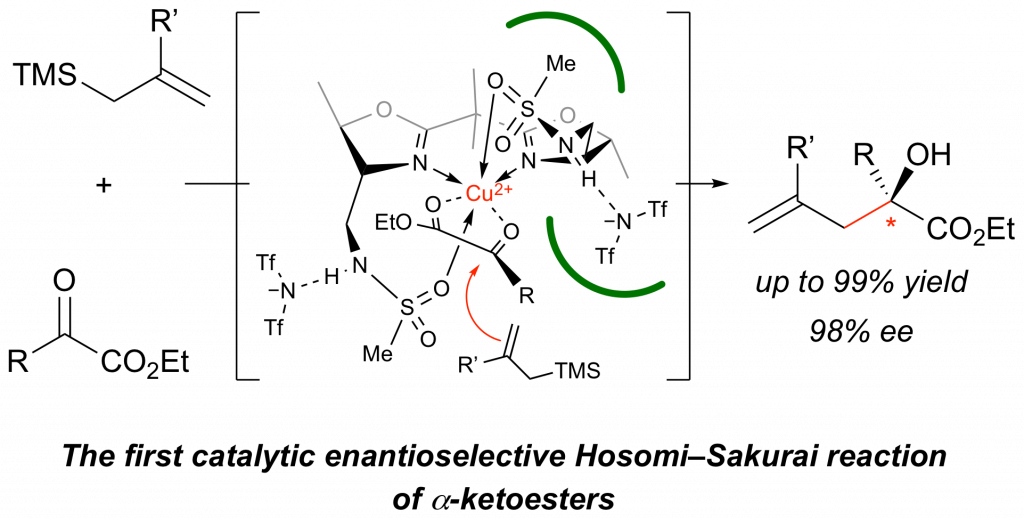
A catalytic enantioselective Hosomi–Sakurai reaction of α-ketoesters has been developed. A copper(II) complex with a chiral bis(oxazoline) ligand bearing methanesulfonamide groups shows excellent catalytic activity to give α,α-disubstituted α-hydroxyesters in high yields with high enantioselectivities. This is the first successful method for the catalytic enantioselective 1,2-addition of α-ketoesters with allylic silanes.
Chiral Pyrophosphoric Acid Catalysts for the para-Selective and Eanantioselective Aza-Friedel–Crafts Reaction of Phenols
Haruka Okamoto, Kohei Toh, Takuya Mochizuki, Hidefumi Nakatsuji, Akira Sakakura,* Manabu Hatano,* Kazuaki Ishihara*
Synthesis 2018, 50 (23), 4577–4590. (Published online: Aug 22, 2018)
DOI: 10.1055/s-0037-1610250 

Chiral BINOL-derived pyrophosphoric acid catalysts were developed and used for the regio- and enantioselective aza-Friedel–Crafts reaction of phenols with aldimines. ortho/ para-Directing phenols could react at the para-position selectively with moderate to good enantioselectivities. Moreover, the gram-scale transformation of a product into the key intermediate for the antifungal agent ( R)-bifonazole was demonstrated.
Enantioselective aza-Friedel–Crafts reaction of furan with α-ketimino esters induced by a conjugated double hydrogen bond network of chiral bis(phosphoric acid) catalysts
Manabu Hatano, Haruka Okamoto, Taro Kawakami, Kohei Toh, Hidefumi Nakatsuji, Akira Sakakura,* Kazuaki Ishihara*
Chem. Sci. 2018, 9 (30), 6361–6367. (Published online: June 25, 2018)
DOI: 10.1039/C8SC02290A 

Chiral C2- and C1-symmetric BINOL-derived bis(phosphoric acid) catalysts, which have OP(=O)(OH)2/OP(=O)(OH)(OR) moieties at the 2,2′-positions, were developed and used for the enantioselective aza-Friedel–Crafts reaction of 2-methoxyfuran with α-ketimino esters for the first time. The intramolecular conjugated double hydrogen bond network is a key to increasing the Brønsted acidity and preventing deactivation of the catalysts. Highly functionalized α-amino acid derivatives with a chiral quaternary carbon center could be transformed into versatile optically active N- and O-heterocycles and an α-aryl-substituted serine.
2017年度
Regioselective DMAD-insertion Reaction of Silyl Dienol Ether of γ-Pyrone under Catalyst- and Heating-Free Conditions
Ichiro Hayakawa,* Yuji Yamanaka, Koichi Mitsudo, Hiromi Ota, Akira Sakakura*
Heterocycles 2017, 94 (12), 2299–2306. (Published online: Oct 26, 2017)
DOI: 10.3987/COM-17-13820 
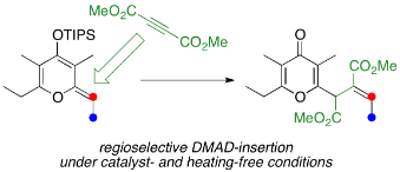
The reaction of silyl dienol ether of γ-pyrone with dimethyl acetylenedicarboxylate (DMAD) gives the regioselective insertion product in 66% yield. This DMAD-insertion reaction is thought to include a three-step sequence: (1) thermal [2+2]-type cycloaddition reaction of silyl dienol ether of γ-pyrone with DMAD, (2) ring-opening electrocyclic reaction of the cyclobutene skeleton, and (3) hydrolysis of the silyl dienol ether. The present reaction proceeds under mild conditions without any catalysts or heating. In addition, the [2+2]-type cycloaddition reaction proceeds regioselectively at the C3–C4 double bond in the silyl dienol ether of γ-pyrone.
Diastereodivergent Henry Reaction for the Stereoselective Construction of Nitrogen-Containing Tetrasubstituted Carbons: Application to Total Synthesis of Manzacidins A and C
Takayuki Kudoh, Yuya Araki, Natsumi Miyoshi, Mizuho Tanioka, and Akira Sakakura*
Asian J. Org. Chem. 2017, 6 (12), 1760–1763. (Published online: Oct 19, 2017)
DOI: 10.1002/ajoc.201700568 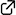
β,β-Disubstituted β-amino alcohols has been synthesized in a diastereodivergent manner from chiral secondary nitroalkanes as starting materials. In this key Henry reaction, the use of different protecting groups resulted in the diastereoselective construction of the tetrasubstituted carbon stereocenter with different configuration. Based on this methodology, a total synthesis of manzacidins A and C has been achieved.
Heat Versus Basic Conditions: Intramolecular Dehydro-Diels–Alder Reaction of 1-Indolyl-1,6-heptadiynes for the Selective Synthesis of Substituted Carbazoles
Takayuki Kudoh, Syo Fujisawa, Megumi Kitamura, Akira Sakakura*
Synlett 2017, 28 (16), 2189–2193. (Published online: July 6, 2017)
DOI: 10.1055/s-0036-1588461
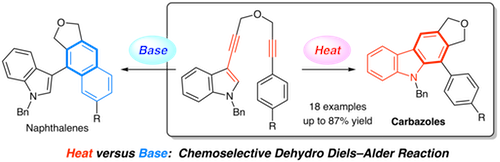
The intramolecular dehydro-Diels–Alder reaction of 1-indolyl-1,6-heptadiynes proceeds smoothly under rather mild heating conditions to give substituted carbazoles in moderate to good yields. The reaction of 7-aryl-1-indolyl-1,6-heptadiynes under heating gives the corresponding carbazoles chemoselectively in high yields, whereas the reaction under basic conditions gives naphthalenes as major products.
Reinvestigation of the Biomimetic Cyclization of 3,5-Diketoesters: Application to the Total Synthesis of Cyercene A, an α-Methoxy-γ-Pyrone-Containing Polypropionate
Kai Onda, Ichiro Hayakawa, Akira Sakakura*
Synlett 2017, 28 (13), 1596–1600. (Published online: Apr 26, 2017)
DOI: 10.1055/s-0036-1588795 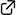
The biomimetic cyclization of 3,5-diketoesters was reinvestigated for the synthesis of α-methoxy-γ-pyrones. 3,5-Diketoesters were selectively synthesized via the aldol reaction of commercially available methyl 2-methyl-3-oxopentanoate with an aldehyde followed by the oxidation with AZADOL® and PhI(OAc)2. The DBU-promoted intramolecular transesterification of 3,5-diketoesters showed excellent reactivity in MeOH, to give the corresponding γ-hydroxy-α-pyrones in high yields under mild reaction conditions. Based on the present cyclization scheme, the total synthesis of cyercene A was achieved.
Copper(II) Triflimide
Akira Sakakura, Kazuaki Ishihara
In e-EROS (Encyclopedia of Reagents for Organic Synthesis); Wiley, 2017, Chapter April 2017, pp. 1–4. (Published online: Apr 10, 2017)
DOI: 10.1002/047084289X.rn01746 
2016年度
Discovery of O6-benzyl glaziovianin A, a potent cytotoxic substance and a potent inhibitor of α,β-tubulin polymerization
Ichiro Hayakawa,* Shuya Shioda, Takumi Chinen, Taisei Hatanaka, Haruna Ebisu, Akira Sakakura, Takeo Usui,* Hideo Kigoshi*
Bioorg. Med. Chem. 2016, 24 (21), 5639–5645. (Published online: Oct 11, 2016)
DOI: 10.1016/j.bmc.2016.09.026 
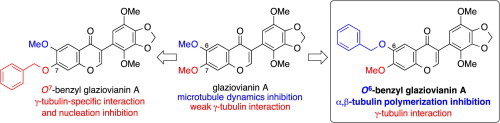
We have discovered O6-benzyl glaziovianin A, which showed stronger inhibition of microtubule polymerization ( IC50 = 2.1 μM) than known α,β-tubulin inhibitors, such as colchicine and glaziovianin A. Also, we performed competition binding experiments of O6-benzyl glaziovianin A and revealed that O6-benzyl glaziovianin A binds to the colchicine binding site with high affinity. It is interesting that glaziovianin A derivatives change their mode of action in benzylation at the O6 (α,β-tubulin inhibitor) or O7 (γ-tubulin-specific inhibitor) position.
A short access to 3,5-disubstituted piperazinones based on the aza-Michael addition of α-amino esters to β-substituted nitroalkenes
Takayuki Kudoh,* Seiji Isoyama, Sachiko Kagimoto, Katsutoshi Kurihara, Akira Sakakura*
Tetrahedron Lett. 2016, 57 (42), 4693–4696. (Published online: Sept 16, 2016)
DOI: 10.1016/j.tetlet.2016.09.015 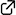
A simple procedure for the synthesis of chiral 3,5-disubstituted piperazinones is described. The aza-Michael addition of α-amino esters to β-substituted nitroalkenes in an organic/aqueous biphasic solvent system followed by reduction of a nitro group with zinc nanopowder in acidic media and intramolecular ester-amide exchange under heating conditions gives piperazinones in good overall yields. This novel three-step process can provide a short access to a variety of chiral 3,5-disubstituted piperazinones simply by changing the combination of starting nitroalkenes and α-amino esters. This process can be applied to the concise synthesis of the piperazinone-containing natural product 6’,6’’-didebromo- cis-3,4-dihydrohamacanthin B.
Enantioselective Bromocyclization of 2-Gernaylphenols Induced by Chiral Phosphite-Urea Bifunctional Catalysts
Yasuhiro Sawamura, Yoshihiro Ogura, Hidefumi Nakatsuji, Akira Sakakura* and Kazuki Ishihara*
Chem. Commun. 2016, 52 (36), 6068–6071. (Published online: Mar 18, 2016)
DOI: 10.1039/C6CC00229C 

Chiral phosphite-urea bifunctional catalysts have been developed for the first enantioselective bromocyclization of 2-geranylphenols with N-bromophthalimide (NBP). The chiral triaryl phosphite moiety activates NBP to generate a bromophosphonium ion. On the other hand, the urea moiety interacts with a hydroxyl group of the substrate through hydrogen bonding interactions. Enantioselectivity is effectively induced through two-point attractive interactions between the catalyst and substrate.
2015年度
Stereoselective Electrophilic Cyclization
Akira Sakakura* and Kazuaki Ishihara*
Chem. Rec. 2015, 15 (4), 728–742. (Published online: July 6, 2015)
DOI: 10.1002/tcr.201500005 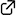
Electrophilic cyclizations of unactivated alkenes play highly important roles in the synthesis of useful building blocks. This account describes our contributions to the rational design of monofunctionalized chiral Lewis base catalysts for enantioselective iodo- and protocyclizations. For the stereoselective promotion of electrophilic bromocyclizations, nucleophilic phosphite–urea cooperative catalysts have been designed.
2014年度
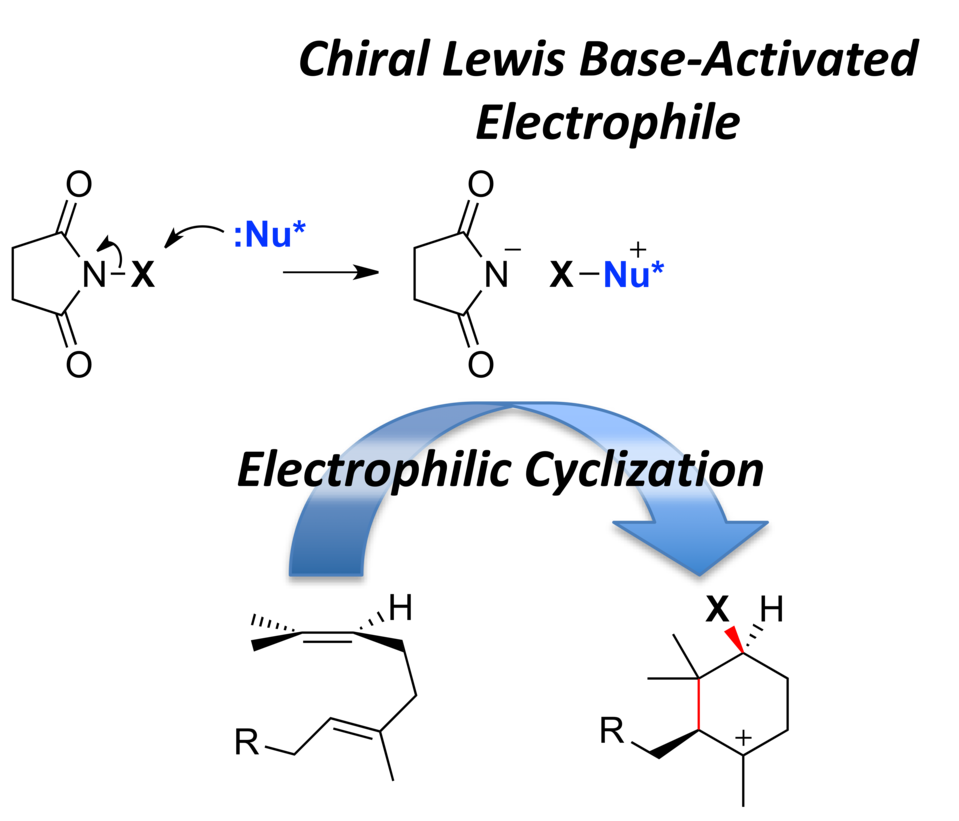
Enantioselective 1,3-Dipolar Cycloaddition of Azomethine Imines with Propioloylpyrazoles Induced by Chiral π−Cation Catalysts
Masahiro Hori, Akira Sakakura,* Kazuaki Ishihara*
J. Am. Chem. Soc. 2014, 136 (38), 13198–13201. (Published online: Sept 16, 2014)
DOI: 10.1021/ja508441t 
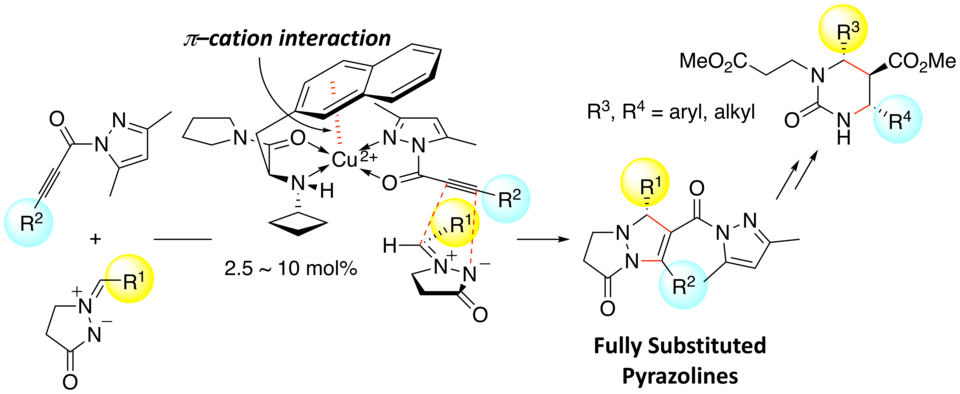
We developed 1,3-dipolar cycloadditions of azomethine imines with propioloylpyrazoles catalyzed by a chiral copper(II) complex of 3-(2-naphthyl)-l-alanine amide. The asymmetric environment created by intramolecular π–cation interaction and the N-alkyl group of the chiral ligand gives the corresponding adducts in high yields with excellent enantioselectivity. This is the first successful method for the catalytic enantioselective 1,3-dipolar cycloaddition of azomethine imines with internal alkyne derivatives to give fully substituted pyrazolines.
This paper was highlighted in Synfacts 2014, 10 (12), 1288.
Synthesis of Chiral Pyrazolines via Chiral π-Cation Catalysis
DOI: 10.1055/s-0034-1379534 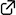
Cooperative Activation with Chiral Nucleophilic Catalysts and N-Haloimides: Enantioselective Iodolactonization of 4-Arylmethyl-4-pentenoic Acids
Hidefumi Nakatsuji, Yasuhiro Sawamura, Akira Sakakura,* Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2014, 53 (27), 6974–6977. (Published online: 19 May 2014)
DOI: 10.1002/anie.201400946 
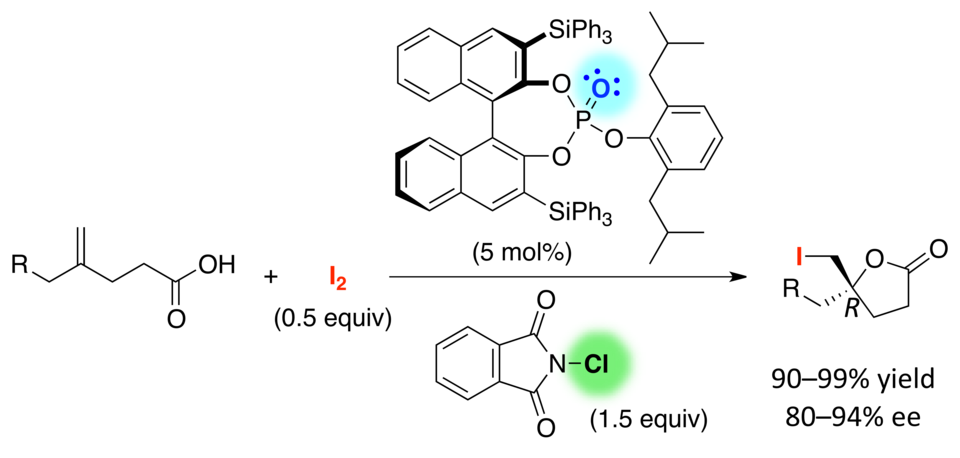
Active duty: Chiral triaryl phosphates promote the enantioselective iodolactonization of 4-substituted 4-pentenoic acids to give the corresponding iodolactones in high yields with high enantioselectivity (see scheme). N-Chlorophthalimide (NCP) is employed as a Lewis acidic activator and oxidant of I2 for the present iodolactonization. In combination with 1.5 equivalents of NCP, only 0.5 equivalents of I2 are sufficient for generating the iodinating reagent.
This paper was highlighted in Synfacts 2014 , 10 (8), 0874.
Enantioselective Iodolactonization by Cooperative Activation
DOI: 10.1055/s-0034-1378508 
Catalytic Enantioselective Inverse Electron Demand Hetero‐Diels–Alder Reaction with Allylsilanes
Yuki Matsumura, Takahiro Suzuki, Akira Sakakura,* Kazuaki Ishihara *
Angew. Chem. Int. Ed. 2014, 53 (24), 6131–6134. (Published online: 29 Apr 2014)
DOI: 10.1002/anie.201402934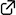
New on the block: The first diastereo‐ and enantioselective title reaction of β,γ‐unsaturated α‐ketoesters with allylsilanes is described. Chiral copper(II) catalysts successfully activate the β,γ‐unsaturated α‐ketoesters and promote the reaction with allylsilanes with excellent enantioselectivities. This process represents a new entry to chiral oxanes, which are useful building blocks. Tf=trifluoromethanesulfonyl.
5.9 Intermolecular Diels–Alder Reactions
Kazuaki Ishihaira, Akira Sakakura
In Comprehensive Organic Synthesis, 2nd Edition, Vol. 5, Gary A. Molander and Paul Knochel (eds.), Oxford: Elsevier; 2014, pp. 351–408.
5.10 Hetero-Diels–Alder Reactions
Kazuaki Ishihaira, Akira Sakakura
In Comprehensive Organic Synthesis, 2nd Edition, Vol. 5, Gary A. Molander and Paul Knochel (eds.), Oxford: Elsevier; 2014, pp. 409–465.
2013年度
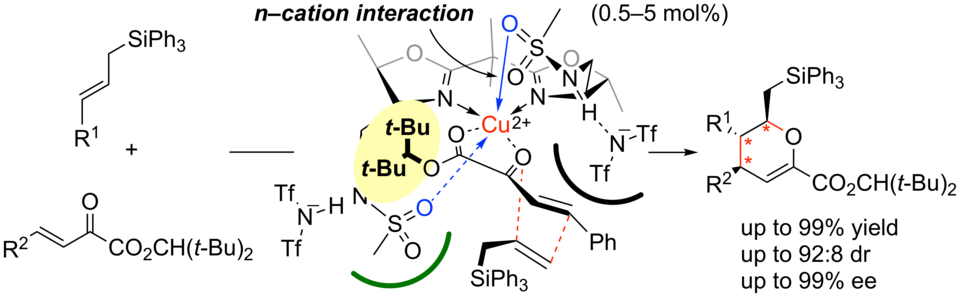
Selective Bromocyclization of 2-Geranylphenols Promoted by Phosphite-Urea Cooperative Catalysts
Yasuhiro Sawamura, Hidefumi Nakatsuji, Matsujiro Akakura, Akira Sakakura,* Kazuaki Ishihara*
Chirality 2014, 26 (7), 356–360. (Published online: 07 Feb 2014)
DOI: 10.1002/chir.22297 
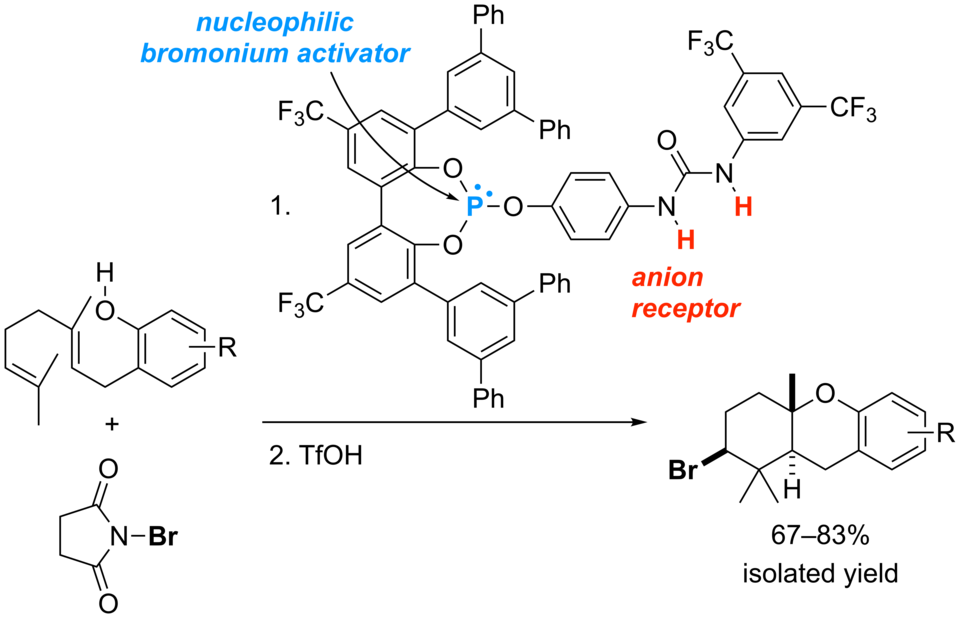
Nucleophilic phosphite-urea cooperative catalysts are highly efficient for the bromonium-induced cyclization of 2-geranylphenols. Phosphite-N,N’-dimethylurea catalysts also show moderate activity, probably due to the steric effect of their bent conformation.
“Phosphite–Urea” Cooperative High-Turnover Catalysts for the Highly Selective Bromocyclization of Homogeranylarenes
Yasuhiro Sawamura, Hidefumi Nakatsuji, Akira Sakakura* and Kazuaki Ishihara*
Chem. Sci. 2013, 4 (11), 4181–4186. (Published online: 02 Aug 2013)
DOI: 10.1039/C3SC51432C 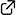
Nucleophilic phosphite-urea cooperative high-turnover catalysts have been designed for the highly selective bromocyclization of homogeranylarenes. The introduction of a urea moiety and bulky aryl groups in the catalyst inhibits decomposition of the catalyst and the generation of byproducts. Only 0.5 mol% of the catalyst successfully promotes the bromocyclization of 4-homogeranyltoluene to give the desired product in 96% yield.
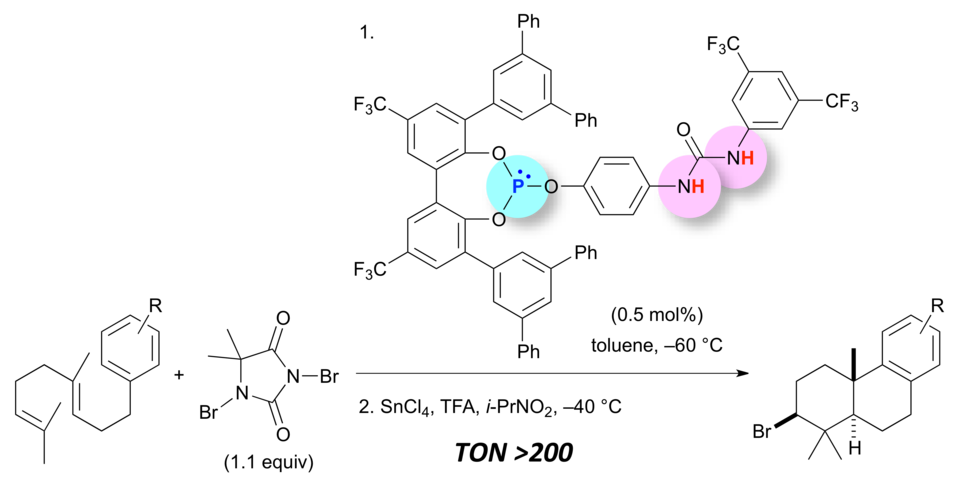
Enantioselective Cyanoethoxycarbonylation of Isatins Promoted by a Lewis Base–Brønsted Acid Cooperative Catalyst
Yoshihiro Ogura, Matsujiro Akakura, Akira Sakakura,* Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2013, 52 (32), 8299–8303. (Published online: 03 July 2013)
DOI: 10.1002/anie.201303572 
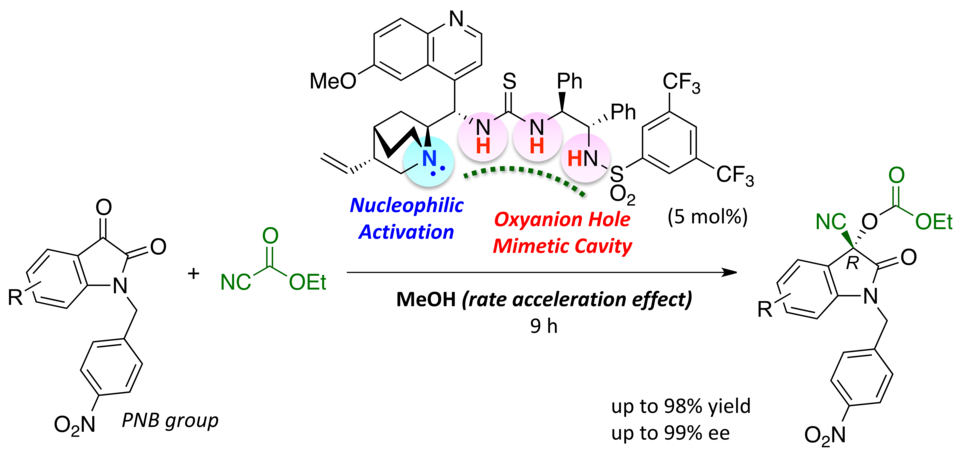
Teaming up to make it happen: In the title reaction, the Lewis basic site of the catalyst activated ethyl cyanoformate, and the deep and flexible Brønsted acidic cavity stabilized and selectively recognized the key reaction intermediate to promote asymmetric acylation (see scheme).
Primary Alkylboronic Acids as Highly Active Catalysts for the Dehydrative Amide Condensation of α-Hydroxycarboxylic Acids
Risa Yamashita, Akira Sakakura,* Kazuaki Ishihara*
Org. Lett. 2013, 15 (14), 3654–3657. (Published online: 26 June 2013)
DOI: 10.1021/ol401537f 

Primary alkylboronic acids such as methylboronic acid and butylboronic acid are highly active catalysts for the dehydrative amide condensation of α-hydroxycarboxylic acids. The catalytic activities of these primary alkylboronic acids are much higher than those of the previously reported arylboronic acids. The present method was easily applied to a large-scale synthesis, and 14 g of an amide was obtained in a single reaction.
Kinetic Resolution of Racemic Carboxylic Acids through Asymmetric Protolactonization Promoted by Chiral Phosphonous Acid Diester
Masayuki Sakuma, Akira Sakakura,* Kazuaki Ishihara*
Org. Lett. 2013, 15 (11), 2838–2841. (published online: May 15, 2013)
DOI: 10.1021/ol401313d 
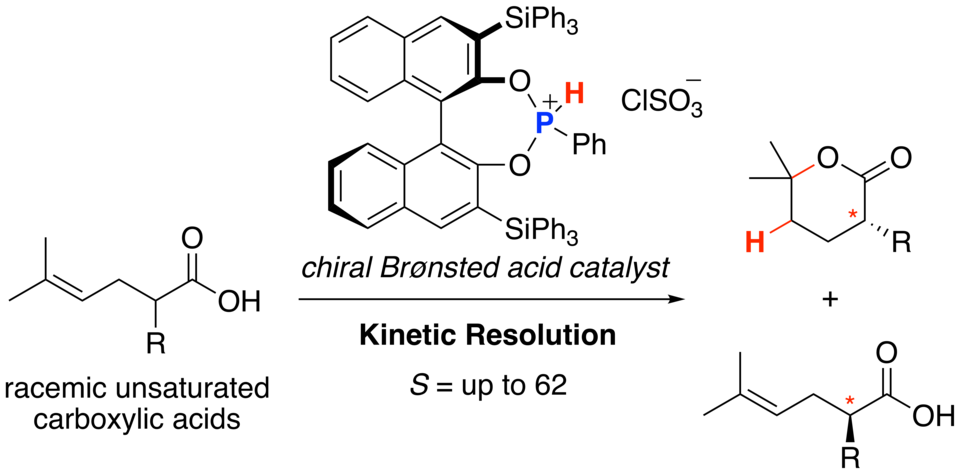
Chiral phosphoniumsalts induce the kinetic resolution of racemicR-substituted unsaturated carboxylic acids through asymmetric protolactonization. Both the lactones and the recovered carboxylic acids are obtained with high enantioselectivities and high S (= kfast/kslow) values . Asymmetric protolactonization also leads to the desymmetrization of achiral carboxylic acids. Notably, chiral phosphonous acid diester not only induced the enantioselectivity but also promoted protolactonization.
This paper was highlighted in Synfacts 2013, 9 (8), 894.
Kinetic Resolution of Unsaturated Carboxylic Acids via Protolactonization
DOI: 10.1055/s-0033-1339441