Thioureas as Highly Active Catalysts for Biomimetic Bromocyclization of Geranyl Derivatives
Miyuki Terazaki, Kei-ichi Shiomoto, Haruki Mizoguchi, Akira Sakakura*
Org. Lett.2019, 21 (7), 2073–2076. (Published online: March 12, 2019)
DOI: 10.1021/acs.orglett.9b00352 
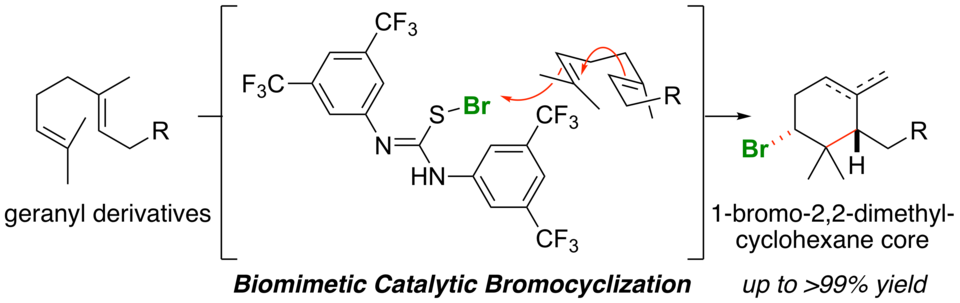
Thioureas bearing electron-deficient aryl groups show high catalytic activity in the biomimetic bromocyclization of geranyl derivatives. The reaction of geranyl derivatives with N-bromosuccinimide (NBS) proceeds rapidly in CH2Cl2 to give the corresponding bromocyclization products in high yields as a ca. 1:1 mixture of endo- and exo-isomers. The reactivity of geranyl derivatives highly depends on the terminal substituent: electron-donating substituents increase the reactivity, while electron-withdrawing substituents decrease the reactivity.