A short access to 3,5-disubstituted piperazinones based on the aza-Michael addition of α-amino esters to β-substituted nitroalkenes
Takayuki Kudoh,* Seiji Isoyama, Sachiko Kagimoto, Katsutoshi Kurihara, Akira Sakakura*
Tetrahedron Lett.2016, 57 (42), 4693–4696. (Published online: Sept 16, 2016)
DOI: 10.1016/j.tetlet.2016.09.015 
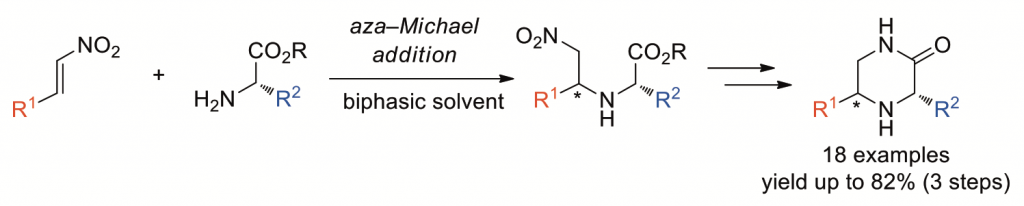
A simple procedure for the synthesis of chiral 3,5-disubstituted piperazinones is described. The aza-Michael addition of α-amino esters to β-substituted nitroalkenes in an organic/aqueous biphasic solvent system followed by reduction of a nitro group with zinc nanopowder in acidic media and intramolecular ester-amide exchange under heating conditions gives piperazinones in good overall yields. This novel three-step process can provide a short access to a variety of chiral 3,5-disubstituted piperazinones simply by changing the combination of starting nitroalkenes and α-amino esters. This process can be applied to the concise synthesis of the piperazinone-containing natural product 6’,6’’-didebromo- cis-3,4-dihydrohamacanthin B.