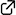Kinetic Resolution of Racemic Carboxylic Acids through Asymmetric Protolactonization Promoted by Chiral Phosphonous Acid Diester
Masayuki Sakuma, Akira Sakakura,* Kazuaki Ishihara*
Org. Lett.2013, 15 (11), 2838–2841. (published online: May 15, 2013)
DOI: 10.1021/ol401313d 
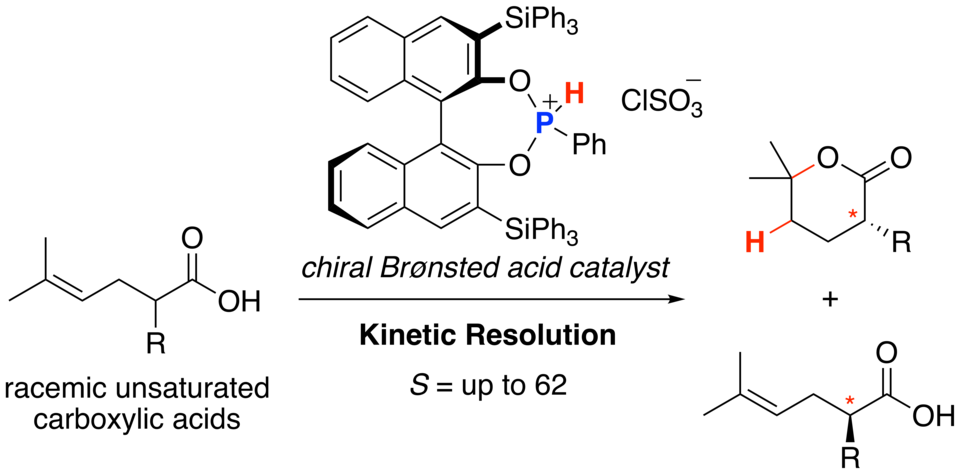
Chiral phosphoniumsalts induce the kinetic resolution of racemicR-substituted unsaturated carboxylic acids through asymmetric protolactonization. Both the lactones and the recovered carboxylic acids are obtained with high enantioselectivities and high S (= kfast/kslow) values . Asymmetric protolactonization also leads to the desymmetrization of achiral carboxylic acids. Notably, chiral phosphonous acid diester not only induced the enantioselectivity but also promoted protolactonization.
This paper was highlighted in Synfacts 2013, 9 (8), 894.
Kinetic Resolution of Unsaturated Carboxylic Acids via Protolactonization
DOI: 10.1055/s-0033-1339441