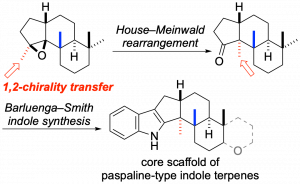
Enantioselective construction of β-hydroxy-α,α-disubstituted α-amino acid derivatives via direct aldol reaction of α-imino esters
Yuya Araki, Masato Hanada, Yoshiko Iguchi, Haruki Mizoguchi, Akira Sakakura*
Tetrahedron, in Press
DOI: 10.1016/j.tet.2022.132695
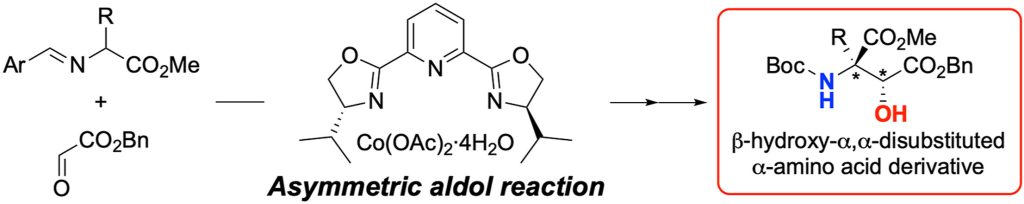
The β-hydroxy-α,α-disubstituted α-amino acid is a valuable structural motif for research in the field of bioorganic chemistry and in the development of peptide drugs. This report describes the enantioselective direct-aldol reaction of α-imino esters with glyoxylate esters. We discovered that a catalytic amount of Co(OAc)2-pybox complex catalyzed the aldol reaction of salicylaldehyde-derived α-imino esters with benzyl glyoxylate in good yield and enantioselectivity. In addition, hydrolysis of the imine moiety of the aldol products followed by Boc protection of the resultant amino group gave the N-Boc-protected amino acid derivatives.