Discovery of O6-benzyl glaziovianin A, a potent cytotoxic substance and a potent inhibitor of α,β-tubulin polymerization
Ichiro Hayakawa,* Shuya Shioda, Takumi Chinen, Taisei Hatanaka, Haruna Ebisu, Akira Sakakura, Takeo Usui,* Hideo Kigoshi*
Bioorg. Med. Chem.2016, 24 (21), 5639–5645. (Published online: Oct 11, 2016)
DOI: 10.1016/j.bmc.2016.09.026 
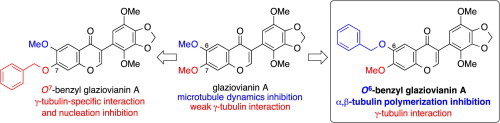
We have discovered O6-benzyl glaziovianin A, which showed stronger inhibition of microtubule polymerization ( IC50 = 2.1 μM) than known α,β-tubulin inhibitors, such as colchicine and glaziovianin A. Also, we performed competition binding experiments of O6-benzyl glaziovianin A and revealed that O6-benzyl glaziovianin A binds to the colchicine binding site with high affinity. It is interesting that glaziovianin A derivatives change their mode of action in benzylation at the O6 (α,β-tubulin inhibitor) or O7 (γ-tubulin-specific inhibitor) position.