Kinetic Resolution of α-Nitrolactones by Catalytic Asymmetric Hydrolysis or Ester–Amide Exchange Reaction
Ryota Nakao, Yudai Fujii, Ichiro Hayakawa, Haruki Mizoguchi, Akira Sakakura*
Synlett2020, 31 (20), 2018–2022. (Published online: October 8, 2020)
DOI: 10.1055/s-0040-1707303 
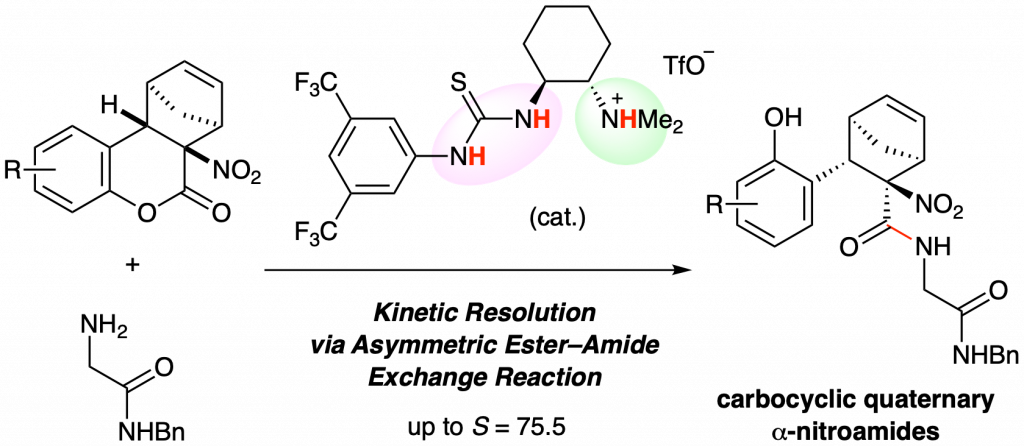
C1-Symmetric chiral ammonium salt catalysts induced a kinetic resolution of racemic α-nitrolactones through an asymmetric ester–amide exchange reaction. The corresponding amides were obtained with high enantioselectivities and high S (= kfast/ kslow) values. This reaction system is a useful approach for obtaining carbocyclic quaternary α-nitroamides as chiral building blocks.