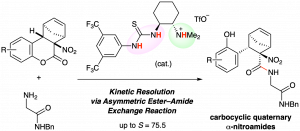
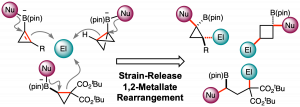
Synthesis of functionalized cyclopropylboronic esters based on a 1,2-metallate rearrangement of cyclopropenylboronate
Haruki Mizoguchi,* Masaya Seriua, Akira Sakakura*
Chem. Commun.2020, 56 (99), 15545–15548. (Published online: November 18, 2020)
DOI: 10.1039/d0cc07134j 

A procedure converting tribromocyclopropane to densely functionalized β-selenocyclopropylboronic ester using the 1,2-metallate rearrangement of a boron ate-complex has been developed. Treatment of an in situ-generated cyclopropenylboronic ester ate-complex with phenylselenenyl chloride triggered stereospecific rearrangement to produce functionalized cyclopropanes. DFT calculations for 1,2-metallate rearrangement suggested that the reaction proceeds through a seleniranium intermediate.