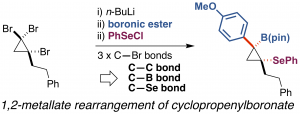
Strain-Release Difunctionalization of C–C σ- and π-bonds of an Organoboron Ate-Complex through 1,2-Metallate Rearrangement
Haruki Mizoguchi,* Akira Sakakura*
Chem. Lett.2021, 50 (4), 792–799. (Published online: January 13, 2021)
DOI: 10.1246/cl.200926
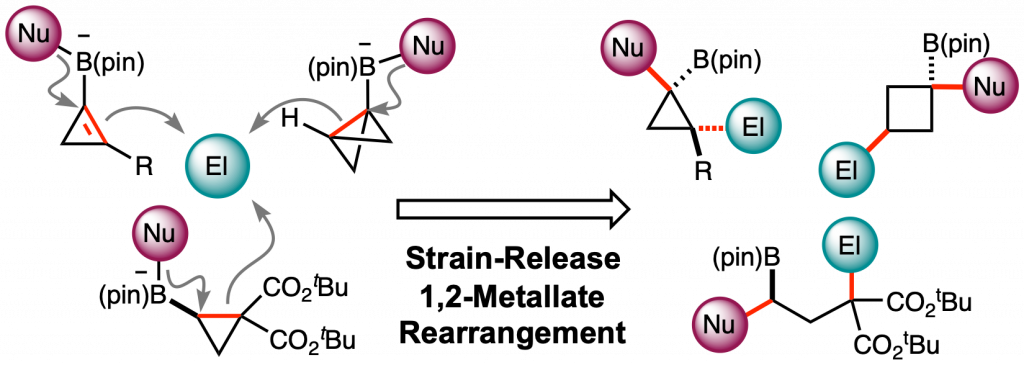
This highlight review describes the recent development of an electrophile-triggered 1,2-metallate rearrangement of organoboronic ester ate-complex, which proceeds through 1,2-difunctionalization of carbon–carbon σ- and π-bonds, using strain energy as a driving force. Coupling reactions of small ring carbocyclic boronic esters, such as cyclopropyl-, bicyclo[1.1.0]butyl-, and cyclopropenyl-boronic ester, are summarized along with the proposed reaction mechanisms and representative examples.