Toward the Synthesis of SB-203207: Construction of Four Contiguous Nitrogen-Containing Stereogenic Centers
Ichiro Hayakawa,* Anna Nagayasu, Akira Sakakura*
J. Org. Chem.2019, 84 (23), 15614–15623. (Published online: November 8, 2019)
DOI: 10.1021/acs.joc.9b02627
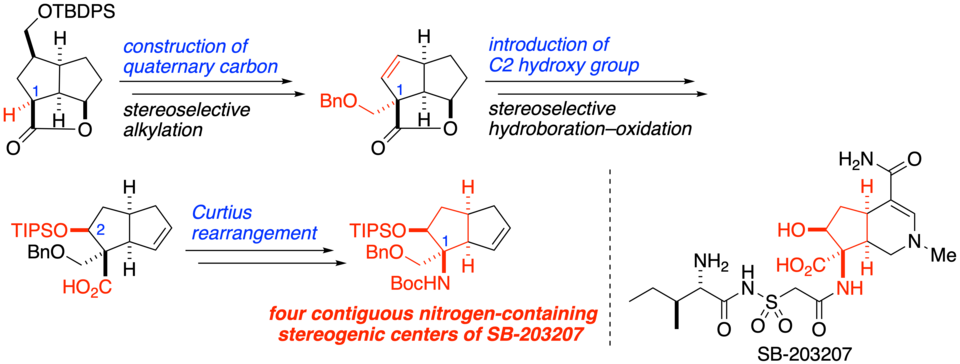
SB-203207 is an altemicidin-type alkaloid that potently inhibits isoleucyl tRNA synthetase activity. Its main structural feature is a hexahydro-6-azaindene framework containing a unique β-hydroxy α,α-disubstituted α-amino acid moiety on the cyclopentane portion. Herein we have established a method for constructing the four contiguous nitrogen-containing stereogenic centers of SB-203207 by using as key steps the stereoselective alkylation of bowl-shaped tricyclic lactone to construct quaternary carbon at C1, the stereoselective hydroboration–oxidation reaction to install the C2 hydroxy group, and the Curtius rearrangement to introduce a nitrogen atom onto the C1 quaternary carbon.