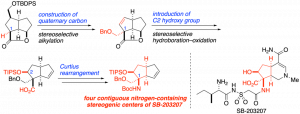
Formal Total Synthesis of Manzacidin B via Sequential Diastereodivergent Henry Reaction
Yuya Araki, Natsumi Miyoshi, Kazuki Morimoto, Takayuki Kudoh, Haruki Mizoguchi, Akira Sakakura*
J. Org. Chem.2020, 85 (2), 798–805. (Published online: December 18, 2019)
DOI: 10.1021/acs.joc.9b02811

A formal total synthesis of manzacidin B is described. β,β-Disubstituted γ-hydroxy-β-aminoalcohol, the key structure of manzacidin B, is stereoselectively constructed via sequential Henry reactions. By taking advantage of noncovalent interactions, such as intramolecular hydrogen bonding and chelation, we could diastereodivergently control the stereoselectivity of the Henry reaction.