Enantioselective aza-Friedel–Crafts reaction of furan with α-ketimino esters induced by a conjugated double hydrogen bond network of chiral bis(phosphoric acid) catalysts
Manabu Hatano, Haruka Okamoto, Taro Kawakami, Kohei Toh, Hidefumi Nakatsuji, Akira Sakakura,* Kazuaki Ishihara*
Chem. Sci.2018, 9 (30), 6361–6367. (Published online: June 25, 2018)
DOI: 10.1039/C8SC02290A 

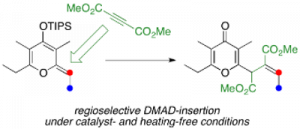
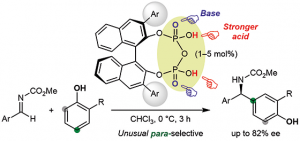
Chiral C2- and C1-symmetric BINOL-derived bis(phosphoric acid) catalysts, which have OP(=O)(OH)2/OP(=O)(OH)(OR) moieties at the 2,2′-positions, were developed and used for the enantioselective aza-Friedel–Crafts reaction of 2-methoxyfuran with α-ketimino esters for the first time. The intramolecular conjugated double hydrogen bond network is a key to increasing the Brønsted acidity and preventing deactivation of the catalysts. Highly functionalized α-amino acid derivatives with a chiral quaternary carbon center could be transformed into versatile optically active N- and O-heterocycles and an α-aryl-substituted serine.