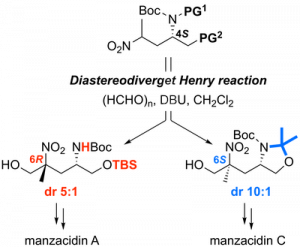
Regioselective DMAD-insertion Reaction of Silyl Dienol Ether of γ-Pyrone under Catalyst- and Heating-Free Conditions
Ichiro Hayakawa,* Yuji Yamanaka, Koichi Mitsudo, Hiromi Ota, Akira Sakakura*
Heterocycles2017, 94 (12), 2299–2306. (Published online: Oct 26, 2017)
DOI: 10.3987/COM-17-13820 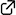
The reaction of silyl dienol ether of γ-pyrone with dimethyl acetylenedicarboxylate (DMAD) gives the regioselective insertion product in 66% yield. This DMAD-insertion reaction is thought to include a three-step sequence: (1) thermal [2+2]-type cycloaddition reaction of silyl dienol ether of γ-pyrone with DMAD, (2) ring-opening electrocyclic reaction of the cyclobutene skeleton, and (3) hydrolysis of the silyl dienol ether. The present reaction proceeds under mild conditions without any catalysts or heating. In addition, the [2+2]-type cycloaddition reaction proceeds regioselectively at the C3–C4 double bond in the silyl dienol ether of γ-pyrone.