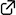Enantioselective 1,3-Dipolar Cycloaddition of Azomethine Imines with Propioloylpyrazoles Induced by Chiral π−Cation Catalysts
Masahiro Hori, Akira Sakakura,* Kazuaki Ishihara*
J. Am. Chem. Soc.2014, 136 (38), 13198–13201. (Published online: Sept 16, 2014)
DOI: 10.1021/ja508441t 
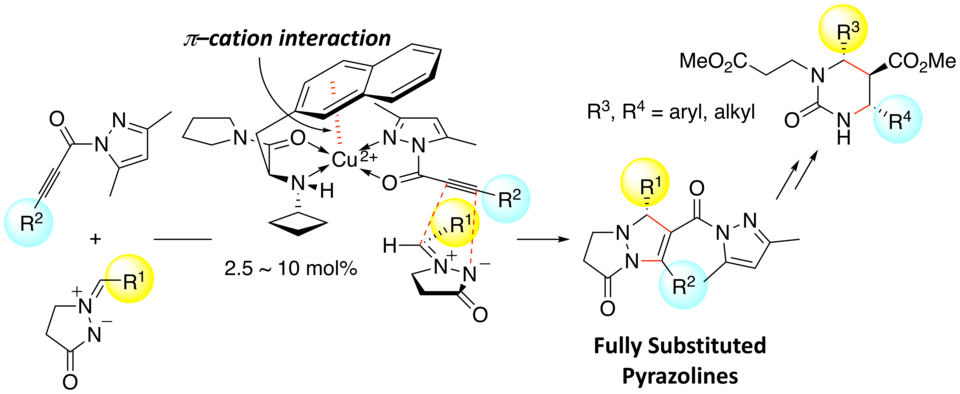
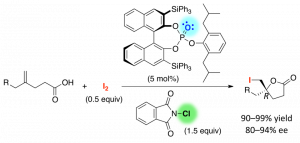
We developed 1,3-dipolar cycloadditions of azomethine imines with propioloylpyrazoles catalyzed by a chiral copper(II) complex of 3-(2-naphthyl)-l-alanine amide. The asymmetric environment created by intramolecular π–cation interaction and the N-alkyl group of the chiral ligand gives the corresponding adducts in high yields with excellent enantioselectivity. This is the first successful method for the catalytic enantioselective 1,3-dipolar cycloaddition of azomethine imines with internal alkyne derivatives to give fully substituted pyrazolines.
This paper was highlighted in Synfacts2014, 10 (12), 1288.
Synthesis of Chiral Pyrazolines via Chiral π-Cation Catalysis
DOI: 10.1055/s-0034-1379534