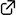Cooperative Activation with Chiral Nucleophilic Catalysts and N-Haloimides: Enantioselective Iodolactonization of 4-Arylmethyl-4-pentenoic Acids
Hidefumi Nakatsuji, Yasuhiro Sawamura, Akira Sakakura,* Kazuaki Ishihara*
Angew. Chem. Int. Ed.2014, 53 (27), 6974–6977. (Published online: 19 May 2014)
DOI: 10.1002/anie.201400946 
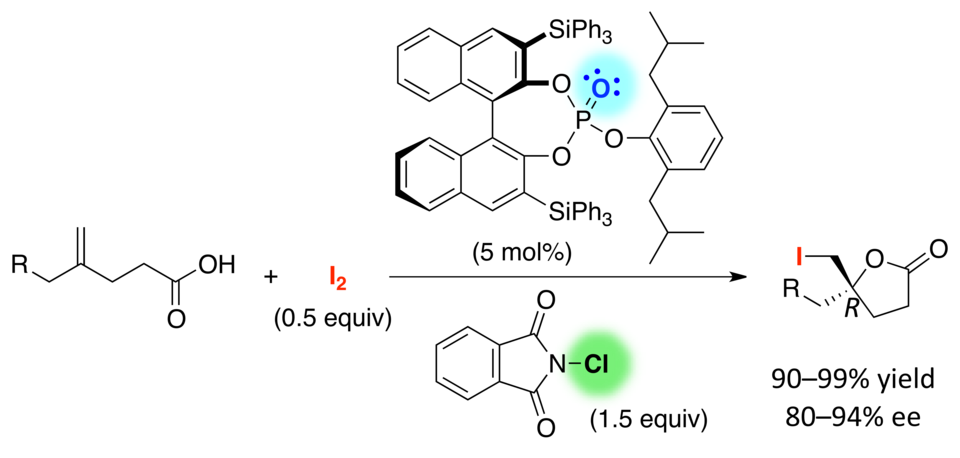
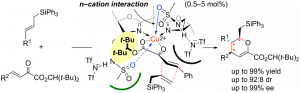
Active duty: Chiral triaryl phosphates promote the enantioselective iodolactonization of 4-substituted 4-pentenoic acids to give the corresponding iodolactones in high yields with high enantioselectivity (see scheme). N-Chlorophthalimide (NCP) is employed as a Lewis acidic activator and oxidant of I2 for the present iodolactonization. In combination with 1.5 equivalents of NCP, only 0.5 equivalents of I2 are sufficient for generating the iodinating reagent.
This paper was highlighted in Synfacts2014 , 10 (8), 0874.
Enantioselective Iodolactonization by Cooperative Activation
DOI: 10.1055/s-0034-1378508