Enantioselective Diels–Alder Reaction of 3-Nitrocoumarins Promoted by Chiral Organoammonium Salt Catalysts
Yudai Fujii, Ryota Nakao, Saki Sugihara, Keita Fujita, Yuya Araki, Takayuki Kudoh, Ichiro Hayakawa, Haruki Mizoguchi, Akira Sakakura*
Synlett2020, 31 (20), 2013–2017. (Published online: October 7, 2020)
DOI: 10.1055/s-0040-1707302 
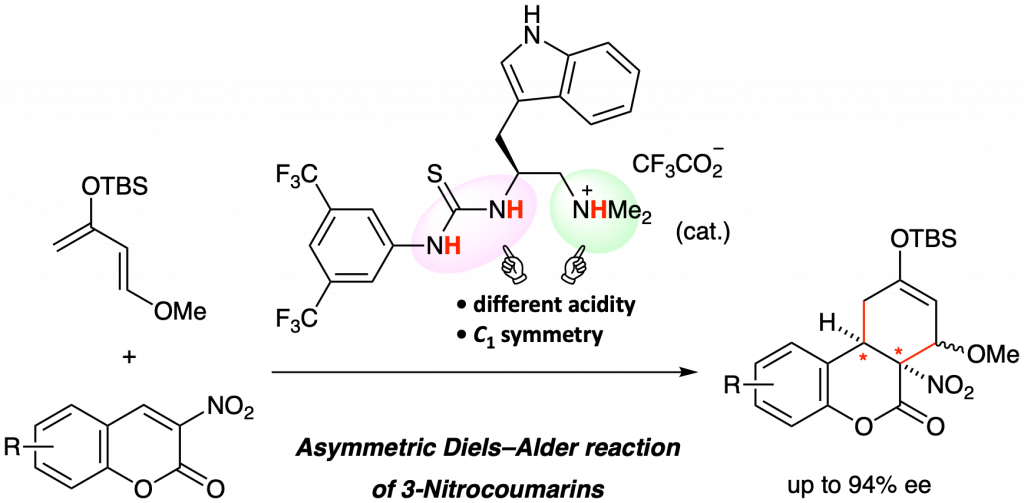
An enantioselective Diels–Alder reaction of 3-nitrocoumarins has been developed. A tryptophan-derived C1-symmetric organoammonium thiourea catalyst promoted the reaction of 3-nitrocoumarins with Danishefsky’s diene to give the corresponding adducts with good enantioselectivity (up to 94% ee). One of the resulting adducts was converted into a chiral carbocyclic quaternary β-amino alcohol.
▶︎ Front Cover